Researchers reveal an added layer of nuance in our sense of smell
The delicate fragrance of jasmine is a delight to the senses. The sweet scent is popular in teas, perfumes and potpourri. But take a whiff of the concentrated essential oil, and the pleasant aroma becomes almost cloying. Indeed, part of the flower’s smell comes from the compound skatole, a prominent component of fecal odor.
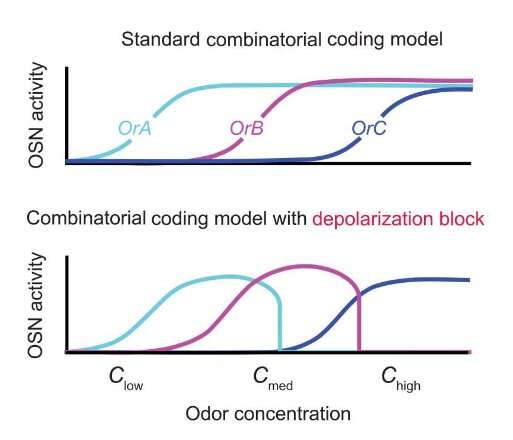
Our sense of smell is clearly a complex process; it involves hundreds of different odorant receptors working in concert. The more an odor stimulates a particular neuron, the more electrical signals that neuron sends to the brain. But researchers at UC Santa Barbara discovered that these neurons actually fall silent when an odor rises above a certain threshold. Remarkably, this was integral to how the brain recognized each smell. “It’s a feature; it’s not a bug,” said Matthieu Louis, an associate professor in the Department of Molecular, Cellular, and Developmental Biology.
The paradoxical finding, published in Science Advances, shakes up our understanding of olfaction. “The same odor can be represented by very different patterns of active olfactory sensory neurons at different concentrations,” Louis said. “This might explain why some odors can be perceived as very different to us at low, medium and very high concentrations. Consider for instance the smell of a ripe banana from a distance (sweet and fruity) versus up-close (overpowering and artificial).”
Humans have several million sensory neurons in our noses, and each of these has one type of odorant receptor. Altogether, we have about 400 different types of receptors with overlapping sensitivity. Each chemical compound is like a different shoe that the receptor is trying on. Some shoes fit snugly, some fit well, while others don’t fit at all. A better fit produces a stronger response from the receptor. Increasing an odor’s concentration recruits neurons with receptors that have are less sensitive to that substance. Our brain uses the combination of activated neurons to distinguish between odors.
Scientists thought that neurons would effectively max out above certain odor concentrations, at which point their activity would plateau. But the team led by Louis’ graduate student, David Tadres, found the exact opposite: Neurons actually fall silent above a certain level, with the most sensitive ones dropping off first.
A simple model
Fruit fly larvae make an ideal model for studying olfaction. They have as many types of odorant receptors as the number of sensory neurons—namely, 21. This one-to-one correspondence makes it simple to test what each neuron is doing.
For the study, Tadres examined larvae with a mutation that entirely eliminated their sense of smell. He then selectively turned that sense back on in a single sensory neuron, enabling the larvae to detect only odors that activated that specific receptor. He placed them next to an odor source and watched.
Even with a single functioning olfactory channel, the larvae could still move toward the stronger smell. But remarkably, they stopped a certain distance away from the source, and just circled it in a fixed orbit. Tadres repeated the experiment with a neuron slightly less sensitive to the odor he was testing, and found that the larvae got closer to the source before stopping.
Puzzled by this behavior, Tadres used electrodes to measure the activity of the sensory neuron. As expected, signaling increased as the odor became more concentrated. But rather than plateau above a certain level, the activity crashed to zero. That’s why the mutant larvae circled the odor source; above a certain concentration, the smell simply disappeared.
“The silencing of the olfactory sensory neuron could easily explain the circling behavior, which was mysterious before,” Tadres said. “From there it wasn’t hard to extrapolate that the current view of how odors are encoded at different concentrations needed to be updated.”
Researchers knew that excessive stimulation can cause nerves to go silent, an effect called “depolarization block.” However, the consensus was that this sort of overload doesn’t occur under natural, healthy conditions. Indeed, this response is associated with issues like epilepsy when it occurs in the central brain. But when Tadres observed it affecting the larvae’s behavior, he suspected that it wasn’t merely an artifact of the experiment.
A mathematical model
Tadres and Louis began investigating the cause of the depolarization block. For assistance, they reached out to Professor Jeff Moehlis, chair of the mechanical engineering department, and Louis’ doctoral student Philip Wong (co-advised by Moehlis), who started constructing a mathematical model of the system.
The voltage across a neuron’s membrane can be described by a system of equations. This model was a breakthrough finding in 1952, and earned a Nobel Prize for its discoverers, Alan Hodgkin and Andrew Huxley. For this case study, Wong added a mathematical representation of the odorant receptor, the “trigger” that initiates the rest of the model. He also included a modification from the field of epilepsy research wherein high stimulation turns off certain ion channels in the cell membrane, preventing a neuron from firing.
Wong’s model was able to fit and predict Tadres’ measurements of the neuron’s electrical activity. “This was quite useful because the electrophysiology data was difficult to collect and very time consuming to analyze,” Wong said.
In addition to corroborating the experimental results, the model is guiding the the team as they continue investigating this effect. “This model may tell us exactly how each neuron is responding to different odors,” Wong said.
The model’s success points to a possible source of the depolarization block: a specific ion channel present in neurons across the animal kingdom. If true, this suggests that most sensory neurons might fall silent following strong and sustained stimulation. The team hopes to validate this hypothesis in an upcoming study.
What’s more, the model predicted that the system would behave differently going up from low odor concentrations versus coming down from high concentrations. Measuring the voltage of the larvae’s neurons confirmed this. When going down, the neuron did not reactivate below the threshold where it had fallen silent. In fact, it largely remained silent until the odor concentration came back down to zero before returning to normal activity.
A better system for smell
This study demonstrated that high odor concentrations can silence the most sensitive receptors. This counterintuitive result marks a fundamental shift in our understanding of smell. “As you increase the concentration of an odor, you’ll start recruiting more and more odorant receptors that aren’t as sensitive to that compound,” Louis explained. “And so, the common view until our work was that you just kept adding active odorant receptors to the picture.”
This makes sense, until you consider the system as a whole. If this were the case, then a compound should activate pretty much all of the receptors above a certain level. “So it would be impossible for you to distinguish between two different odors at very high concentrations,” Tadres said. “And that’s clearly not the case.”
Having certain sensory neurons drop out as others join in might help preserve the distinction between odors at high concentrations. And this could prove important for survival. It might prevent poisons and nutrients that share certain compounds from smelling alike when you take a big whiff of them.
It could also have consequences for how we perceive odors. “We speculate that removing successive high-sensitivity olfactory sensory neurons is like removing the root of a musical chord,” Louis said. “This omission of the root is going to alter the way your brain perceives the chord associated with a set of notes. It’s going to give it a different meaning.”
A subtle floral note suggests an orchard may be in bloom nearby, useful information for a hungry animal. Meanwhile, the same compounds in higher concentrations could produce the pungent ripeness of decaying fruit or even sewage: something best avoided. Studies like this reveal ever more complexities to our sense of smell, which evolved to help us navigate an equally complex chemical landscape.
More information:
David Tadres et al, Depolarization block in olfactory sensory neurons expands the dimensionality of odor encoding, Science Advances (2022). DOI: 10.1126/sciadv.ade7209
Journal information:
Science Advances
Source: Read Full Article
