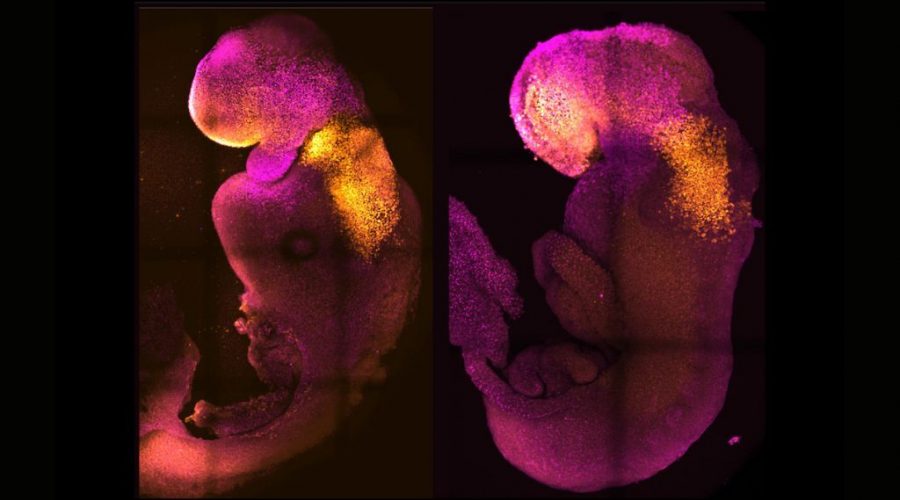
Lab-made mouse embryos grew brains and beating hearts, just like the real thing
Scientists coaxed mouse stem cells to grow into synthetic embryos that began developing hearts and brains, just like the real thing.
The lab-made embryos, crafted without any eggs or sperm and incubated in a device that resembles a fast-spinning Ferris wheel full of tiny glass vials, survived for 8.5 days. That’s nearly half the length of a typical mouse pregnancy. In that time, a yolk sac developed around the embryos to supply nutrition, and the embryos themselves developed digestive tracts; neural tubes, or the beginnings of the central nervous system; beating hearts; and brains with well-defined subsections, including the forebrain and midbrain, the scientists reported in a study published Thursday (Aug. 25) in the journal Nature (opens in new tab).
“This has been the dream of our community for years and [a] major focus of our work for a decade, and finally, we’ve done it,” senior study author Magdalena Zernicka-Goetz, a developmental and stem-cell biologist with labs at the University of Cambridge, UK, and the California Institute of Technology in Pasadena, said in a statement (opens in new tab).
The new work produced very similar results as an earlier study, published Aug. 1 in the journal Cell (opens in new tab), which was led by Jacob Hanna, an embryonic stem cell biologist at the Weizmann Institute of Science in Israel and co-author of the new Nature paper. In their recent Cell study, Hanna’s team used different starting stem cells but the same incubator to culture synthetic mouse embryos for 8.5 days. Those embryos also grew digestive tracts, beating hearts, and tiny, wrinkled brains before ultimately dying, Live Science previously reported.
Although the two recent studies produced similar embryos, the experiments started out slightly differently. In the Cell study, the researchers started by coaxing mouse stem cells into a naive state from which they could morph into any cell type, such as heart, brain or gut cells. From there, the team divided these naive cells into three groups. In one group, they switched on genes to form the placenta, and in another group, they switched on genes to make the yolk sac. The last group they left alone to develop into embryos.
Zernicka-Goetz’s research group, on the other hand, began with three mouse stem cell types, rather than starting with only naive cells. One type of stem cell gave rise to the embryo, while the other two morphed into the placental tissues and yolk sac. Throughout the experiment, they observed how these three stem cell types interacted, exchanging chemical messages and physically butting up against each other in the glass vials.
Studying such exchanges could give hints as to how the earliest stages of embryonic development unfold in humans — and what happens when things go awry.
“This period of human life is so mysterious, so to be able to see how it happens in a dish — to have access to these individual stem cells, to understand why so many pregnancies fail and how we might be able to prevent that from happening — is quite special,” Zernicka-Goetz said. “We looked at the dialogue that has to happen between the different types of stem cell at that time — we’ve shown how it occurs and how it can go wrong.”
—How long can human embryos stay frozen?
—Impeccably preserved dinosaur embryo looks as if it ‘died yesterday’
—Part-human, part-monkey embryos grown in lab dishes
In both the Cell and Nature studies, the resulting synthetic embryos closely resembled natural embryos, albeit with some slight differences and defects in how the tissues self-organized. However, in both experiments, a very low proportion of the stem cells actually gave rise to embryos, suggesting that the efficiency of both systems could be improved. In addition, neither set of synthetic embryos survived to the ninth day of development — an obstacle that would need to be overcome in follow-up studies.
“The reason for the block in further development is unclear but might relate to the defects in the formation of some of the placental cell types that the authors report,” James Briscoe, a principal group leader and assistant research director at the Francis Crick Institute in the U.K. who was not involved in either study, told the Science Media Centre (opens in new tab), a U.K.-based press office that works with researchers, journalists and policymakers to disseminate accurate scientific information.
The research also raises ethical questions about if and how such technology might be applied to human cells in the future.
Originally published on Live Science.
Nicoletta Lanese
Staff Writer
Nicoletta Lanese is a staff writer for Live Science covering health and medicine, along with an assortment of biology, animal, environment and climate stories. She holds degrees in neuroscience and dance from the University of Florida and a graduate certificate in science communication from the University of California, Santa Cruz. Her work has appeared in The Scientist Magazine, Science News, The San Jose Mercury News and Mongabay, among other outlets.
Source: Read Full Article