How pancreatic cancer defies treatment by leveraging a protein
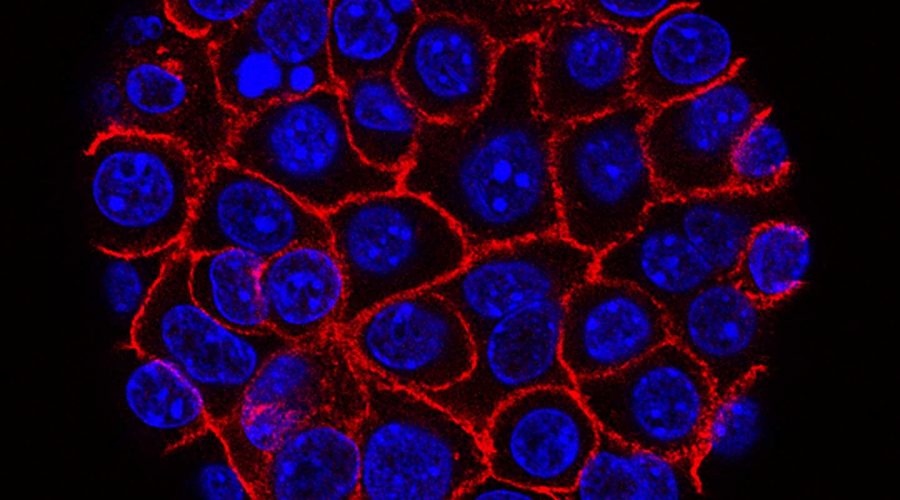
Pancreatic cancer is the third deadliest cancer in the United States, after lung and colorectal, though far less common. It is also among the hardest to effectively treat, with pancreatic cancer stem cells quickly developing resistance to conventional and targeted treatments, such as chemotherapy and emerging immunotherapies. As a result, the 5-year survival rate for people diagnosed with pancreatic cancer is just 10%.
In a new paper, published January 18, 2023 in Nature Communications, an international team of scientists, led by researchers at University of California San Diego School of Medicine and the Sanford Consortium for Regenerative Medicine, reveal another way in which the most-resistant pancreatic cancer cells defy treatment by leveraging a member of a group of proteins that ordinarily might suppress tumors to instead help cancer cells evade therapy and grow more quickly.
Previous research has shown that pancreatic cancer treatment resistance is caused by differing responses to conventional agents, fueled by the heterogeneity (diversity) of tumor cells—and in particular, stem cell characteristics that encourage therapy resistance.
In the new study, senior author Tannishtha Reya, Ph.D., formerly a professor of Pharmacology and Medicine and director of the Division of Cancer Biology at UC San Diego School of Medicine, and colleagues investigated how shifting epigenomics (the multitude of proteins that tell the genome what to do) rather than genomic changes (specific to the genes themselves) might be driving resistance.
“Pancreatic cancer stem cells, which are aggressive cancer cells that can resist conventional therapies and drive tumor relapse, rely upon epigenetic regulation to protect themselves and promote survival and growth,” said Reya, now a professor of Physiology and Cellular Biophysics at Columbia University and associate director of translational research at the Herbert Irving Comprehensive Cancer Center.
“We wanted to identify the underlying tools and mechanisms that cancer stem cells use to better understand treatment resistance—and perhaps how they might be circumvented.”
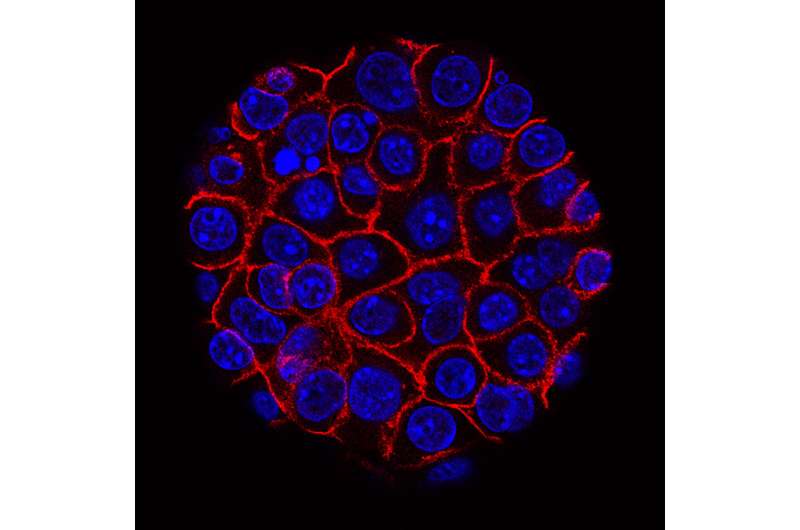
Reya and colleagues zeroed in on SMARCD3, a member of the SWI/SNF family of proteins that regulate chromatin, a mixture of DNA and proteins that form chromosomes and are required for stem cell function in development.
But while SWI-SNF subunits often act as tumor suppressors, the researchers found that SMARCD3 was amplified in cancer, notably abundant in pancreatic cancer stem cells and upregulated or increased in the human disease.
And when researchers deleted SMARCD3 in models of pancreatic cancer, the loss of the protein reduced the growth of tumors and improved survival, especially in the context of chemotherapy.
“Importantly, we found that SMARCD3 helps control lipid and fatty acid metabolism, which are associated with therapy resistance and poor prognosis in cancer,” said Reya.
“Our data suggest that therapy resistant pancreatic cancer cells depend upon SMARCD3 to help ensure a metabolic landscape in which they can avoid anti-cancer treatments and grow aggressively. That makes SMARCD3 an exciting new target for potential therapies.”
More information:
L. Paige Ferguson et al, Smarcd3 is an epigenetic modulator of the metabolic landscape in pancreatic ductal adenocarcinoma, Nature Communications (2023). DOI: 10.1038/s41467-023-35796-7
Journal information:
Nature Communications
Source: Read Full Article