Anatomy of a vaccine: What it takes to create a safe, effective COVID shot
Shawn stepped into the UCLA Vine Street Clinic in Hollywood with confidence. He offered up his arm. The UCLA doctor injected him. It took seconds; there was barely a sting.
Twenty-four hours after the first of two shots, given 28 days apart, he suffered the headaches and fatigue associated with a milder case of COVID-19. But Shawn remained calm, resolved to honor the memory of his mother, a nurse who had died in May 2020 from an unrelated cause.
The 57-year-old nonprofit worker had been thinking about the challenges of COVID-19 for a long time, and he decided to go through the lengthy consent process for the medical trial. “It gave me something to do with my anger that was so much better than yelling at someone for not wearing a mask,” he says. “And [at UCLA] I felt I was in good hands.”
Shawn is one of many volunteers who have stepped up to participate in medical trials at UCLA, which is part of a global network that’s determined to help find a vaccine against the novel coronavirus.
The stakes are huge. More than 250,000 Americans have already died, and there have been more than 1 million deaths around the world. Economies have been brought to their knees, social tensions have disrupted communities and emotional maladies are on the rise.
In response, doctors and scientists have been challenged to be resilient and ingenious. They’re taking an array of different approaches, knowing that public confidence in vaccines hangs in the balance.
In addition, it has been a challenge to create a vaccine in such a short amount of time—similar efforts have taken five to 10 years. Pharmaceutical giant Pfizer and biotech firm Moderna have both reported remarkable progress, announcing in November that their vaccine candidates were more than 90% effective. All of which has raised questions about the next steps, such as how the vaccines will be distributed.
Deploying bacteria to fight a virus
“I don’t want to make a vaccine to protect against mild disease,” says Dr. Marcus Horwitz, distinguished professor of medicine and microbiology, immunology and molecular genetics at the David Geffen School of Medicine at UCLA. “I want to protect people who are going to get severe disease.”
Horwitz has already developed vaccines against the bacteria behind tuberculosis, anthrax and the tick-borne disease tularemia, but he has never tried to create a vaccine against a virus. “When faced with a worldwide pandemic, we thought we might be able to make a contribution,” he says.
Vaccines work by training the immune system to recognize and fight disease-causing pathogens, such as viruses or bacteria. Doctors introduce the body’s immune system to antigens, which are molecules from the virus or bacteria, and the immune system responds by making proteins called antibodies and immunity-building T cells, which both neutralize the pathogen.
The delivery of these antigens requires a delicate calculus: It must provoke the immune system, but not go so far as to make the patient ill. “You need a vector that will wake up the immune system of the host, but not cause any further harm,” Horwitz says.
The vaccine approach by Horwitz and his team, including lead investigator Qingmei Jia, is a medical outlier: They adapted an existing antibacterial “platform” to build protection against SARS-CoV-2, the virus that causes COVID-19. The team has shown that their vaccine candidate protects hamsters, which develop severe disease in a way similar to humans.
Some of the potential vaccines for SARS-CoV-2 use a weakened form of an adenovirus, which causes the common cold, to deliver the S protein that is found on the surface of the SARS-CoV-2 virus. Horwitz’s vaccine stands out from the pack because it uses a weakened bacterium to deliver two SARS-CoV-2 proteins, the M and N proteins.
That difference could have a tremendous impact. Billions of COVID-19 vaccine doses are needed, and bacteria, unlike viruses, are easy and cheap to produce—and transportable.
Boosting a fading immune system
The success of a COVID-19 vaccine also depends on the immune system, which can be less robust in older people.
This is a problem that has driven Song Li, chair of the bioengineering department at the UCLA Samueli School of Engineering, who has focused his career on cell and tissue engineering. Adapting a concept from cancer immunotherapy, Li is developing a biomaterial vaccine booster using artificial cells that could improve the immune system’s ability to generate long-term protection.
When the immune system encounters a destructive pathogen, it produces cells that are designed to attack the invader. A small number of those cells, called T memory stem cells, can stay in the system for years—ready for a future invasion. Unfortunately, our ability to produce T memory stem cells declines as we get older. Li hopes his booster, in combination with a vaccine, can help fragile immune systems effectively fight against the SARS-CoV-2 virus.
“My goal at the outset was to help the elderly population,” Li says. “But it could be useful for any person whose immune system needs help generating protection from the virus.”
Achieving better insights
Another UCLA team—led by Bogdan Pasaniuc, Dr. Manish Butte and Dr. Daniel Geschwind, the Gordon and Virginia MacDonald Distinguished Professor of Human Genetics at the Geffen School of Medicine—is trying to find out why the virus significantly impacts some, but leaves others relatively unscathed.
“We know age is a major factor, but we see older people who get infected and do quite well,” Geschwind says. “We have a limited ability to predict how sick someone will get.” His team hopes that studying whole-genome sequences from thousands of COVID-19 patients will reveal hidden factors that make some more vulnerable than others. The research could help identify people who are at higher risk for infection as well as develop new treatment and prevention strategies.
Dr. Brigitte Gomperts, professor of pediatrics and pulmonary medicine and a member of the UCLA Broad Stem Cell Research Center, is studying how COVID-19 affects lung tissue. By using stem cell–derived clusters of lung cells, known as “organoids,” she can rapidly screen thousands of prospective treatments. Because the organoids are grown from human cells and reflect the cell types and architecture of the lungs, they can offer insights into how the virus infects and damages the organ.
Helping underserved communities
At UCLA medical centers around Los Angeles County, physicians are ensuring that their medical trials include diverse groups of people and women of all ages.
“COVID-19 has hit the African American and Latino communities particularly hard,” says Dr. Jesse Clark, associate professor-in-residence in the department of medicine at the Geffen School of Medicine. “We have to make sure that any vaccine has been determined to be safe and effective in all populations that will receive it.”
Clark is medical director of the UCLA Vine Street Clinic, which is involved in the Moderna clinical trial. Notably, Moderna’s vaccine works differently from a typical vaccine, because it doesn’t contain the virus at all. Instead, it uses messenger RNA, or mRNA, which uses the body’s genetic code to produce antibodies against the virus.
“CNN mentioned that the vaccine trials were having trouble finding minorities to participate,” says Roderick, a 37-year-old IT manager and father of two, who is participating in the Moderna trial. “Being Black and Mexican, and knowing how hard my demographic has been hit, I just went ahead and signed up online. It’s worth doing to help out.”
Meanwhile, Dr. Katya Corado, an infectious disease specialist at Harbor-UCLA Medical Center in Torrance, has been enrolling patients in a phase 3 clinical trial of an adenovirus vector vaccine that’s under development by the University of Oxford and the biopharmaceutical company AstraZeneca.
All vaccines undergo three phases of clinical trials, according to rules set by the Food and Drug Administration. Phase 1, which involves 20 to 100 volunteers, tests the safety and dosage of the vaccine. Phase 2 tests the drug’s efficacy and side effects among several hundred participants, and phase 3 gathers more information about a vaccine’s safety and effectiveness by studying thousands of volunteers.
“In the phase 3 trial, we focus on studying how effective the vaccine is in populations that need it most,” Corado says.
Clark and Corado are both hopeful that their work can protect the most vulnerable, which includes people over 65, patients with chronic conditions, those facing economic disadvantages and essential workers.
Building trust in vaccines
Inoculations have eradicated past epidemics, such as smallpox. But public faith in vaccines has wavered, especially when a now-disproven report in 1998 suggested that the measles, mumps and rubella vaccine was linked to autism spectrum disorder. That has led to U.S. outbreaks of measles, which had been previously eliminated. So scientists recognize the importance of getting the COVID-19 vaccine right.
There are other factors to consider as well. Vaccine distribution will be high on the agenda of the incoming White House administration, but if supply is limited, the Centers for Disease Control and Prevention recommends prioritizing certain groups, such as medical workers.
Also, some vaccines currently in development need to be stored in ultra-cold conditions. For example, Pfizer’s vaccine must be stored at minus 70 degrees Celsius, while Moderna’s vaccine must be kept at minus 20 degrees Celsius—the temperature of a regular freezer. These factors will affect how the vaccines are distributed.
A global community
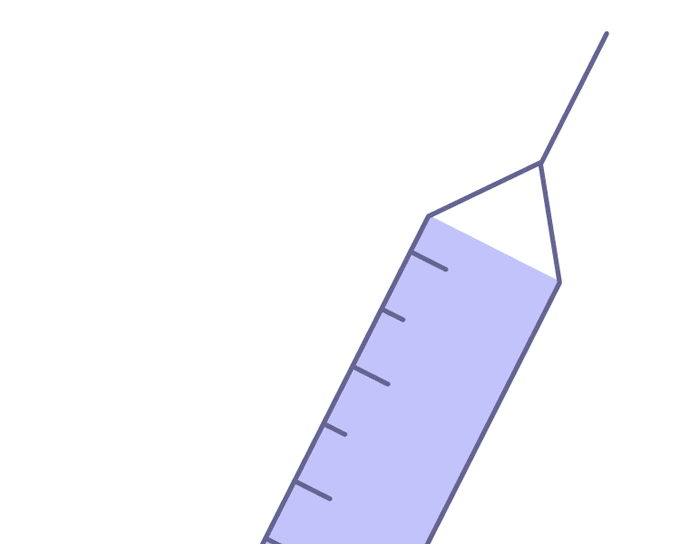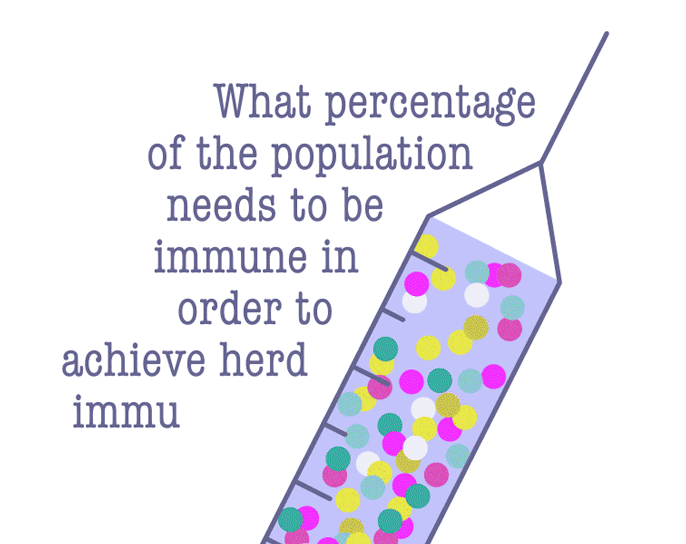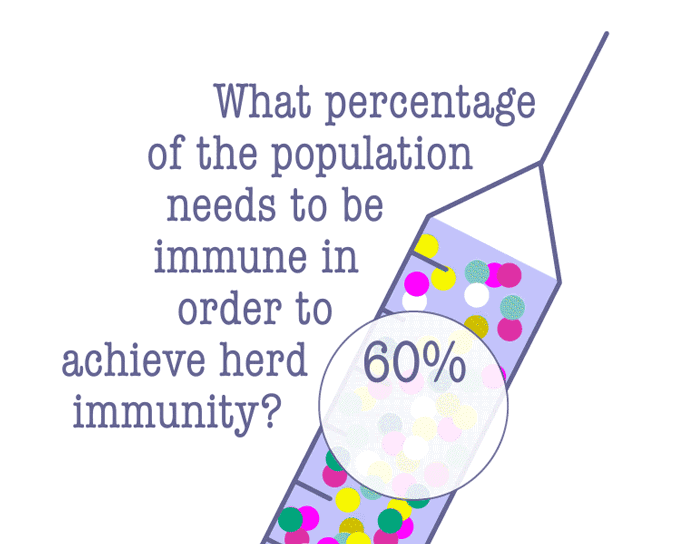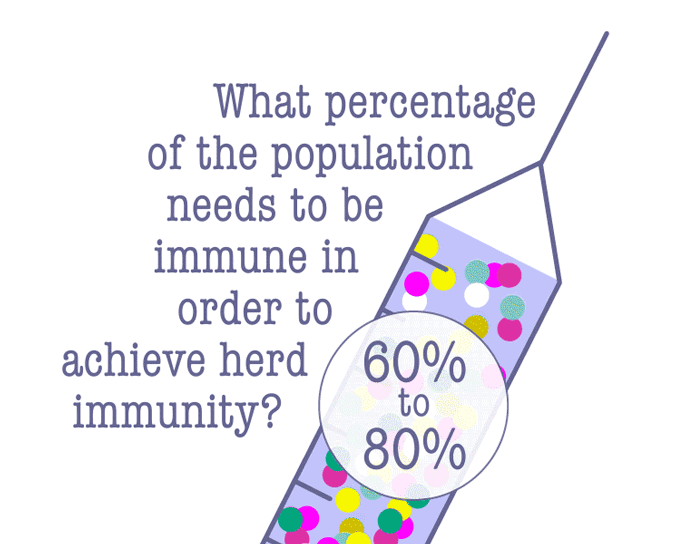
Some lawmakers have advocated letting the virus run its course in the hopes of achieving herd immunity, which is when enough people have become immune to an infectious disease, either through being infected or vaccination. Since the COVID-19 vaccine is still pending, a majority of people will need to be infected in order to achieve herd immunity—and that comes at a terrible cost.
According to Dr. Robert Kim-Farley, professor-in-residence of epidemiology at the UCLA Fielding School of Public Health, up to 2 million Americans would have to die before the country reached herd immunity.
He argues that vaccines work, even if they are not perfectly safe or perfectly effective, as proven by the near-eradication of polio. But approving vaccines prematurely—to buckle under the pressure of politics or profit—could cause a terrible backlash against being vaccinated, which could lead to future outbreaks.
Source: Read Full Article
