Why Cryo-EM is Gaining Popularity in Structural Biology
By Shelley Farrar, MSc, BSc
Cryo-electron microscopy, also known as Cryo-EM, is a technique for visualizing macromolecular structures which has been developed over the last two decades. It is a form of transmission electron microscopy which permits samples to be studied at cryogenic temperatures. Biological structures such as ribosomes and mitochondria from pathogens, ion channels and super-coiled DNA have all been defined in atomic detail via Cryo-EM. Thoroughly visualizing and understanding biological structures is an essential first step for the development of new drugs and treatments. For this reason, the added advantages of Cryo-EM mean the technique is gaining popularity in structural biology.
Imaging biological structures via Cryo-EM
In Cryo-EM, the biological material in a sample is flash frozen by putting thin films of sample into ethane baths that are cooled to the temperature of liquid nitrogen (-180°C). The sample is retained in the frozen solution and bombarded with electrons. The electrons pass through a lens creating a magnified image on the detector, from which the structure of the sample can be analyzed.
There are two main types of Cryo-EM. The first is single particle analysis Cryo-EM where three-dimensional structures are constructed from two-dimensional projections. Two – dimensional images of the same kind of biological object within the frozen structure are captured first and are then organized into three-dimensional structures by image processing algorithms. The second type of Cryo-EM is Cryo-electron tomography. This method involves capturing multiple images of a single biological object by tilting the object at various angles for the electron beam to penetrate, creating a three-dimensional structure.
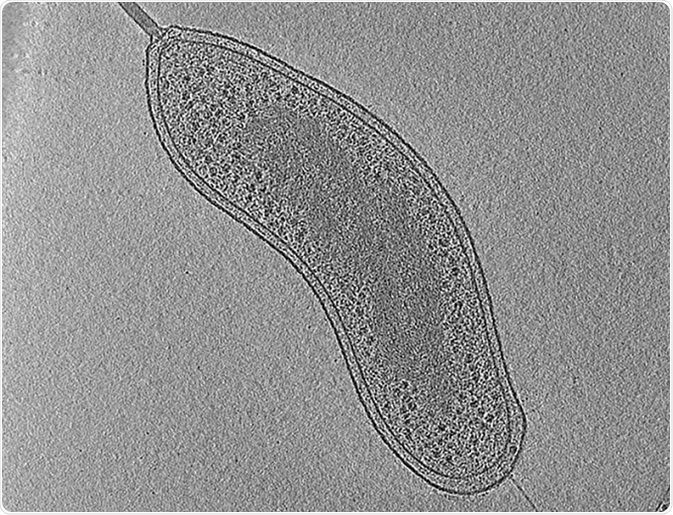
Image: An example of electron cryo-tomography. The image shows a central slice through a tomographic reconstruction of an intact Bdellovibrio bacteriovorus cell. Scale bar 200 nm.©Eikosi/ en.wikipedia.org. Shared under the license.
Advantages of Cryo-EM in structural biology
Previous structural biology techniques included X-ray crystallography and nuclear magnetic resonance spectroscopy. Both methods have had limited application because of the need for large sample sizes. X-ray crystallography also necessitates the crystallization of specimens, a difficult process that changes the environment to one that is non-physiological. Cryo-EM does not require large sample sizes or crystallization and is therefore suited to the visualization of structures at near-atomic resolution. The method also has the advantage of not chemically fixing or staining the specimen, meaning it can be studied within the native physiological environment. Moreover, without the restriction of crystals locking the sample in a static pose, structures can be flash-frozen in several conformations to allow biological mechanisms to be deduced.
Cryo-EM advances through the direct electron detector device
Early Cryo-EM applications were limited because of the low power electron beams that had to be used in order to avoid destroying the sample, creating low resolution images. The development of the direct electron detector device has meant high resolution images can be formed using fewer electrons. The direct electron detector device is a camera with an increased ability to detect electrons allowing quick-fire images of a single molecule at dozens of frames per second. The development of improved image-processing algorithms has also aided in the Cryo-EM revolution. For techniques where a three-dimensional image is reconstructed from two-dimensional images, such algorithms are required to determine image orientation as well as aligning them in order. Improvement in Cryo-EM technology has also lowered the size limit of samples that can be frozen allowing individual proteins to be imaged and analyzed.
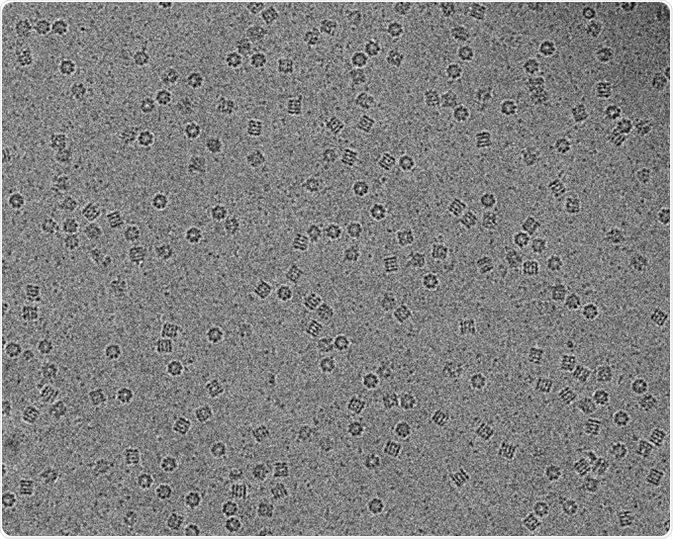
Image: Molecular chaperon GroEL in vitreous ice captured using Cryo-electron microscopy. Scale bar 200 nm. © Vossman/ en.wikipedia.org. Shared under the license.
Recent applications of Cryo-EM
The field of Cryo-EM has gained enough popularity that only certain samples, such as viruses and ribosomes, are occasionally imaged using X-ray crystallography. Cryo-EM has now provided imaging at atomic resolution of the structural changes that occur in the p97 protein. This protein is an important target for cancer drug development as the structure and interactions of the protein are critical for cancer cell activity. Through the advanced imaging abilities of Cryo-EM, the type of p97 inhibitor binding and contact sites have been observed. This study achieved resolutions of 2.3 ångströms, with the unit ångström being the equivalent of 0.1 nanometers. Cryo-EM has the potential for further improvements to resolution with advances in detector technology and sample preparation currently under way.
Sources:
- Kühlbrandt, W. 2014. ‘Microscopy: Cryo-EM enters a new era’, eLife, 3, e03678 https://elifesciences.org/articles/03678 – x20879003
- Gelfand, A. 2016. ‘The rise of the Cryo-electron microscope’, Biomedical Computational Review: biomedicalcomputationreview.org/content/rise-cryo-electron-microscopy
- Nogales, E. 2015. ‘The development of cryo-EM into a mainstream structural biology technique’, Nature Methods, 13, pp. 24-27. http://www.nature.com/nmeth/journal/v13/n1/full/nmeth.3694.html
- Banerjee, S. et al. 2016. ‘Å resolution cryo-EM structure of human p97 and mechanism of allosteric inhibition’, Science, 351, 871-875. http://science.sciencemag.org/content/early/2016/01/28/science.aad7974
Further Reading
- All Microscopy Content
- Advances in Fluorescence Microscopy
- Applications in Light Microscopy
- Electron Microscopy: An Overview
- Brief History of Microscopy
Last Updated: Jun 28, 2019
Source: Read Full Article
