Video app eases methadone dose-confirmation burden
When methadone is given to people who want to break their opioid addiction, federal law requires those patients to initially consume their daily dose in view of opioid treatment program staff. This requirement exists in part to keep methadone from being taken improperly or ending up on the black market. After a patient demonstrates earnest effort in the treatment regimen, it may be eased to allow more at-home dosing.
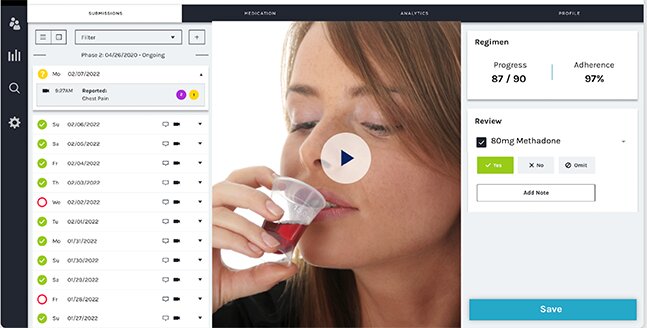
A small study published this month in the Journal of Substance Abuse Treatment suggests that a smartphone-based video app can enable the same validation of dose adherence while relieving patients’ burdens of time and travel.
“Our findings suggest it is feasible for counselors and patients to use this app, which provided confirmation of treatment while allowing them to not come to the clinic daily and to live their lives more fully,” said Dr. Judith Tsui. She is the study’s senior author and a professor of medicine at the University of Washington School of Medicine.
COVID-19 was the catalyst for the pilot study. In early 2020, the federal agency that oversees methadone treatment for addiction offered a waiver to its strict direct-observation requirements in order to minimize the virus’ potential spread via non-essential clinic visits.
“They recognized that it was unsafe for people to go into an opioid-treatment program every day when there were no vaccines available,” said Kevin Hallgren, the study’s lead author and a UW associate professor of psychiatry and behavioral medicine.
The scenario presented a window of opportunity, and in April 2020 the pilot began. Ninety patients were invited to start a two-week trial using the video app, and 60 accepted.
The smartphone-based app, developed by emocha Health, enabled patients to engage with counselors through uploaded videos. At their convenience, patients recorded themselves ingesting their daily dose and uploaded the time-stamped videos to a secure server. Counselors were able to review the submitted videos within 24 hours.
Of the initial 60, 33 were deemed successful at the two-week trial and were invited to extend their participation until the pilot ended. On average, pilot participants had 53 out of 60 days with a methadone dose observed (by video or in person) by a clinician—significantly higher than the matched control group’s 16 days with a methadone dose observed.
“Among those who were offered the program, the majority accepted,” Hallgren said. “The majority of people who continued using the technology graduated to additional take-home doses: They went from getting one week’s worth of take-home methadone to two weeks, or from two weeks to four weeks. They had to come into the clinic less often.”
The technology offered flexibility on the clinical side, too: Counselors could watch videos at their convenience and work from home during COVID restrictions.
Tsui and Hallgren acknowledged the study’s main limitations: The cohort was small, involving only a single opioid-treatment program, and its duration was brief. Although many participants made good use of the video app, no light was shed on its longer-term effectiveness at helping to end their addictions, or its prospective appeal with a broader swath of clinics.
“We think the technology can provide a middle ground, where doses can be observed and confirmed with less hassle and less potential exposure to diseases like COVID-19,” Hallgren said. “We suspect that most patients take their methadone as prescribed, and in these cases, video observation might seem unnecessary. But we also know that a certain percentage of patients don’t always take their medication as directed, which can create concerns for providers and health systems.”
Source: Read Full Article