Targeting epiregulin in treatment-damaged tumor microenvironment restrains therapeutic resistance
A recent study published in Oncogene and led by Dr. Sun Yu’s group at Shanghai Institute of Nutrition and Health (SINH) of the Chinese Academy of Sciences revealed the roles and functional mechanisms of senescence-associated secretory factor epiregulin (EREG) in treatment-damaged tumor microenvironments, providing a new therapeutic target and noninvasive biomarker for treatment of human malignancies including prostate cancer.
Cellular senescence is a unique cellular state with many distinct and unique features. Among these cell characteristics, the unique secretory phenotype of senescent cells, namely senescence associated secretory phenotype (SASP), makes it possible to execute a complex signal transfer function on other cells in the microenvironment.
This secretory phenotype has been found to contribute significantly to the progression of diseases in the elderly, including chronic inflammatory processes. Therefore, the study of SASP associated factors and their functions in tumor and other pathological microenvironments has far-reaching guiding significance for the clinical treatment of geriatric diseases.
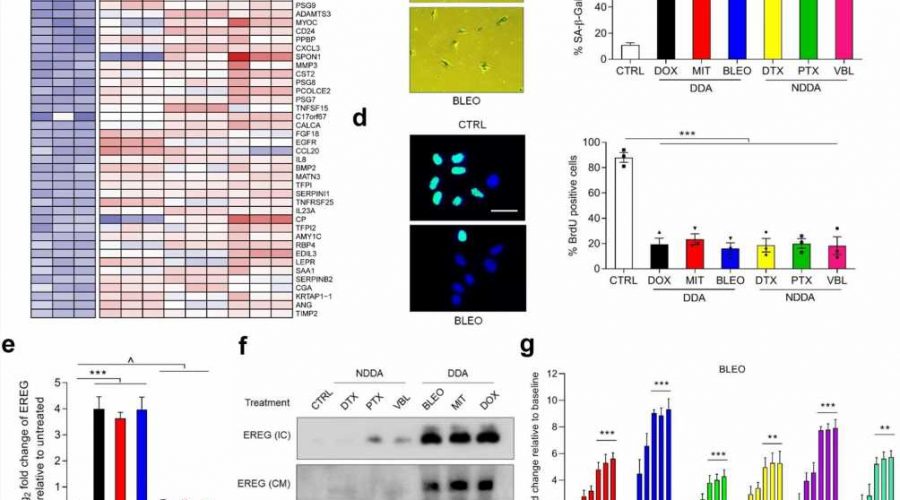
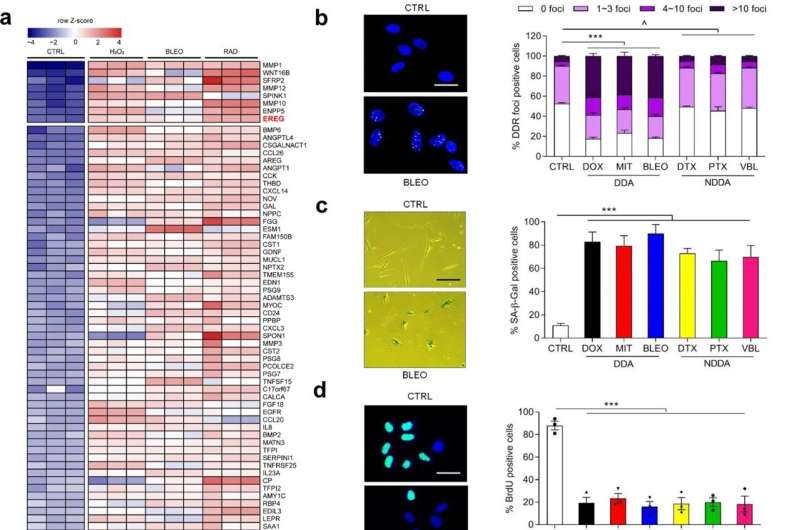
In this study, the researchers confirmed that EREG expression was significantly upregulated under senescence induced conditions of DNA damage type by senescence induction in primary human stromal cells. The analysis of cancer samples from clinical prostate cancer and breast cancer patients before and after chemotherapy showed that EREG was significantly up-regulated in senescent paracancer stromal cells.
In terms of the mechanism, DNA damage induces nuclear localization of the transcription factor NF-κB in stromal cells and binds to multiple sites in the EREG promoter region, thereby contributing to the upregulation of EREG expression after cell aging. Other aging-related factors were also noted to promote EREG transcription. In the tumor microenvironment, EREG activates several signaling pathways by binding to EGFR receptors on the surface of adjacent cancer cells, and induces the aggravation of malignant phenotypes such as proliferation, migration and invasion of cancer cells and the occurrence of tumor drug resistance.
Using RNA-Seq analysis, the researchers identified a ubiquitin ligase, MARCHF4, that was significantly upregulated in EREG-stimulated cancer cells. MARCHF4 can regulate the down-regulation of E-cadherin expression in cancer cells and inhibit the apoptosis of cancer cells, thus leading to the occurrence of drug resistance.
In a mouse model, the combination of EREG monoclonal antibody and EGFR monoclonal antibody significantly reduced tumor volume and significantly prolonged the progression-free period. It is worth mentioning that EREG expression level in clinical cancer patients can effectively and accurately predict the efficacy of cancer treatment.
Source: Read Full Article