Study reveals inaccurate label claims on unregulated CBD products
Recent studies have highlighted the therapeutic benefits of cannabidiol (CBD), which is a non-intoxicating component of Cannabis sativa. Previously, the association of Δ9-tetrahydrocannabinol (Δ9-THC) with CBD limited studies on its medicinal benefits.
The Agricultural Improvement Act of 2018 (2018 Farm Bill) legalized the use of products from the plant parts of Cannabis sativa, which is also known as hemp. This led to the rapid growth of the CBD industry.

Study: Label accuracy of unregulated cannabidiol (CBD) products: measured concentration vs. label claim. Image Credit: IRA_EVVA / Shutterstock.com
Background
In June 2018, a purified oral solution of CBD known as Epidolex® received approval by the United States Food and Drug Administration (FDA) for the treatment of three forms of epilepsy.
However, except for Epidolex®, other CBD products remain unregulated in the U.S. In June 2019, the FDA held a general public hearing to hear concerns from scientists regarding CBD regulation.
Many research groups, along with the FDA, have identified numerous issues with label accuracy for several CBD products. This issue was not restricted to the U.S., as similar reports have also been published in the Netherlands, United Kingdom, and Italy.
A new Journal of Cannabis Research study aimed to determine the CBD content in various products and compare it to their label claims. The products assessed in this study were purchased from several stores in Central Kentucky, as well as online retailers, between April 2, 2021, and May 9, 2021.
About the study
The current study involved a total of 80 samples, 44 of which were purchased from online retailers that were U.S.-based. The remaining 36 samples were purchased from local retailers within Central Kentucky.
Taken together, the samples included in this study were produced by 51 different brands. Epidiolex® was also used as a positive control. All CBD products were tested immediately upon opening and before their expiration dates.
To determine label accuracy, a ±10% of the allowable variance was used. The products with CBD concentration exceeding 110% of the labeled value were considered under-labeled, while those with less than 90% of their labeled CBD content were considered over-labeled. Products with a CBD concentration that was between 90-110% of their labeled CBD content were considered to be accurately labeled.
Thereafter, all samples were prepared and analyzed using liquid chromatography-tandem mass spectrometry (LC-MS/MS).
Study findings
Out of the 80 tested samples, 31% were under-labeled, 54% were labeled accurately, and 15% were over-labeled. Out of the 44 products that were purchased online, 25% were under-labeled, 61% were labeled accurately, and 14% were over-labeled. For the 36 products that were purchased locally, 39% were under-labeled, 17% were over-labeled, and 44% were labeled accurately.
The CBD concentrations ranged from 2.9 to 61.3 mg/mL for all unregulated samples, with labeled CBD concentrations ranging from 17-159%. The average amount of CBD for under-labeled products was 121% of the label claim, as compared to 61% in over-labeled products.
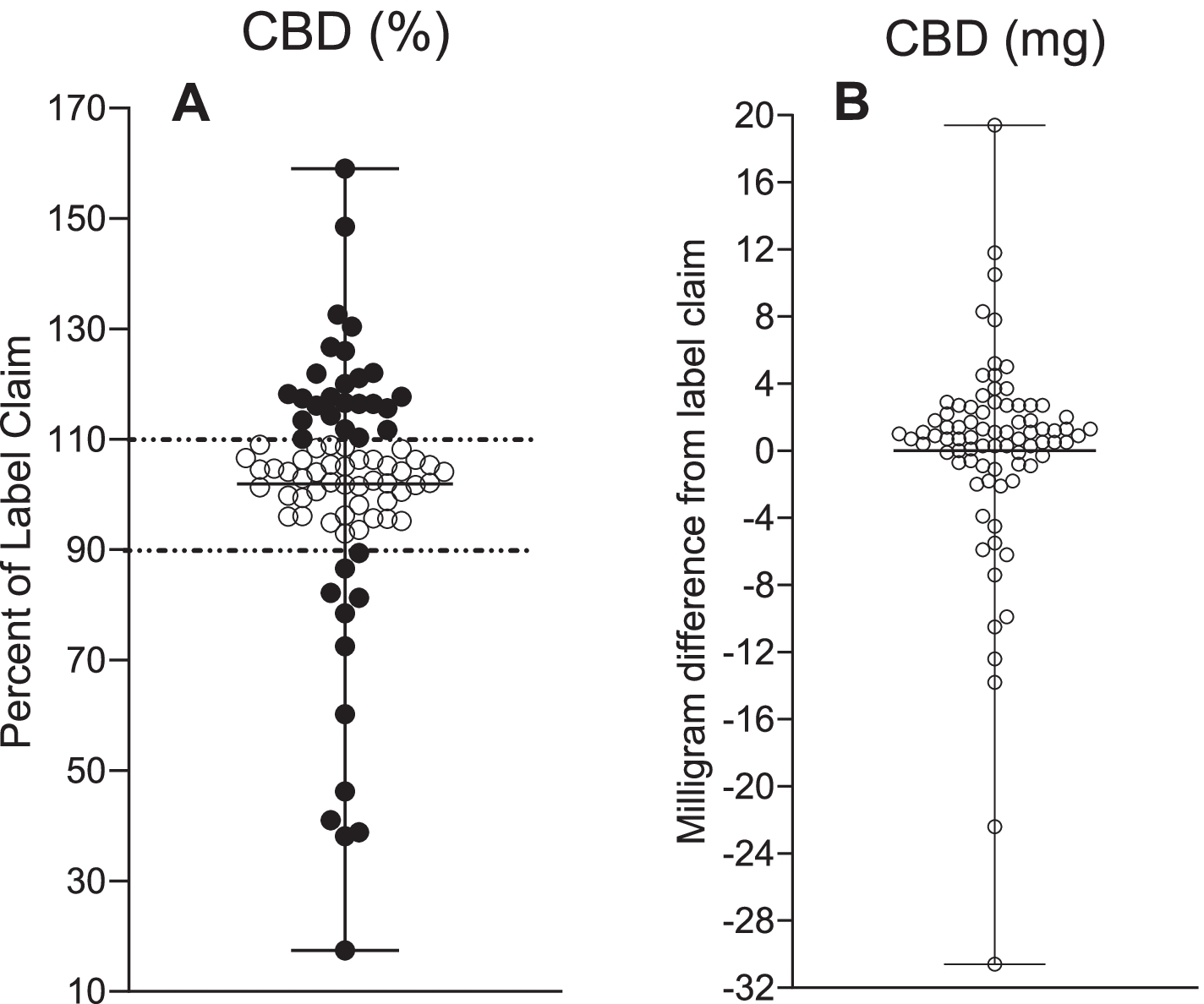 CBD measurements in 80 commercially available CBD oil products and Epidiolex®. A The percentage of CBD label claim content with ± 10% tolerance denoting under-labeling (> 110%) and over-labeling (< 90%). B Deviation from CBD label claim in milligram
CBD measurements in 80 commercially available CBD oil products and Epidiolex®. A The percentage of CBD label claim content with ± 10% tolerance denoting under-labeling (> 110%) and over-labeling (< 90%). B Deviation from CBD label claim in milligram
Conclusions
The current study provides evidence that CBD products from several countries around the world often had inconsistent CBD concentrations with their label claims. Such inaccurate labelings can pose safety risks to consumers who use CBD products for medical treatments.
Thus, clear regulations from both the federal and state agencies, as well as good manufacturing practices, are required to guarantee that the label claims of CBD products are accurate.
Limitations
The study has certain limitations. First, the study included only hemp-derived oil products. Second, only CBD concentrations were reported, while other cannabinoid concentrations were not reported. The current study also did not involve a formal sampling protocol.
- Johnson, E., Kilgore, M., & Babalonis, S. (2022). Label accuracy of unregulated cannabidiol (CBD) products: measured concentration vs. label claim. Journal of Cannabis Research. doi:10.1186/s42238-022-00140-1.
Posted in: Medical Science News | Medical Research News | Miscellaneous News
Tags: Cannabidiol, Cannabinoid, Cannabis, Chromatography, Epilepsy, Food, Hearing, Liquid Chromatography, Manufacturing, Mass Spectrometry, Research, Spectrometry, Tetrahydrocannabinol

Written by
Suchandrima Bhowmik
Suchandrima has a Bachelor of Science (B.Sc.) degree in Microbiology and a Master of Science (M.Sc.) degree in Microbiology from the University of Calcutta, India. The study of health and diseases was always very important to her. In addition to Microbiology, she also gained extensive knowledge in Biochemistry, Immunology, Medical Microbiology, Metabolism, and Biotechnology as part of her master's degree.
Source: Read Full Article
