Noninvasive, ultrasound-based brain biopsy is feasible, safe in people: Study
The blood-brain barrier, the body’s way of shielding sensitive brain tissue from viruses, toxins and other harmful substances in the blood, can pose a problem for physicians caring for patients with suspected brain diseases such as cancer.
Molecular and genetic information would be invaluable for confirming a diagnosis and guiding treatment decisions, but such molecules are normally confined to the brain by the barrier. Neurosurgeons routinely perform surgical brain biopsies to obtain this data on brain tumors, but such procedures carry risks and are not feasible for all tumors or for many other kinds of brain diseases.
Researchers at Washington University in St. Louis have developed an anatomically precise technique called sonobiopsy that uses ultrasound and microbubbles to disrupt the barrier temporarily and allow RNA, DNA and proteins from the brain to spill out into the blood, where they can be detected and analyzed.
The researchers developed and previously tested the technique in animals. In a new study, available online in the journal npj Precision Oncology, they showed that the technique is feasible and safe for use in people, and could open the door to noninvasive biopsies for suspected brain tumors and other brain diseases.
“Magnetic resonance imaging (MRI) revolutionized the field of brain disease diagnosis in the 1980s and ’90s by allowing for structural and functional imaging of the brain,” said Eric C. Leuthardt, MD, the co-senior author on the paper and co-inventor of the technology. Leuthardt is the Shi Hui Huang Professor of Neurosurgery and a professor of neuroscience at the School of Medicine and a professor of biomedical engineering and of mechanical engineering at the McKelvey School of Engineering.
“Sonobiopsy is the third revolution, the molecular revolution. With this technique, we can obtain a blood sample that reflects the gene expression and the molecular features at the site of a lesion in the brain. It’s like doing a brain biopsy without the dangers of brain surgery.”
The technique was pioneered by Leuthardt and Hong Chen, Ph.D., an associate professor of biomedical engineering at McKelvey Engineering and of neurosurgery at the School of Medicine. Leuthardt is the director and Chen a member of the Division of Neurotechnology in the Department of Neurosurgery, which focuses on intensely multidisciplinary research to create innovative engineered solutions that can be translated to patients with neurologic diseases. Washington University owns a patent on the sonobiopsy technology.
The procedure works by using focused ultrasound to target a lesion in the brain with millimeter-scale accuracy, followed by the injection of microbubbles into the bloodstream. The microbubbles travel to the targeted spot and then pop, tearing tiny holes in the blood-brain barrier that close within a few hours, leaving no lasting damage. That window of time is long enough for biomolecules from the lesion to escape into the blood, where they can be collected with an ordinary blood draw.
“We’ve essentially initiated a new field of study for brain conditions,” said Chen, the other co-senior author on the paper and co-inventor of the technology.
“With this capability to noninvasively, nondestructively access every part of the brain, we can get genetic information on tumors before going in surgically, which would help a neurosurgeon determine how best to approach the surgery. If they see something suspicious on imaging, they could confirm whether a tumor is recurring or not. We can now start to interrogate diseases for which surgical biopsies aren’t done, such as neurodevelopmental, neurodegenerative and psychiatric disorders.”
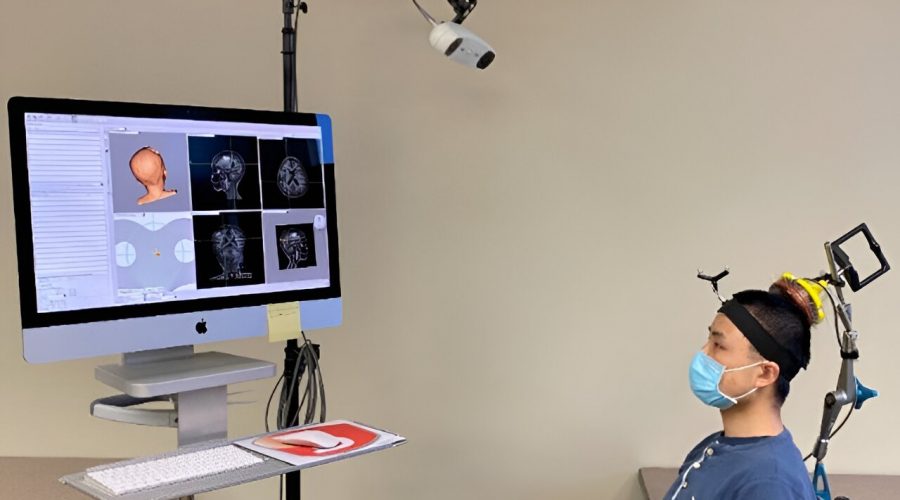
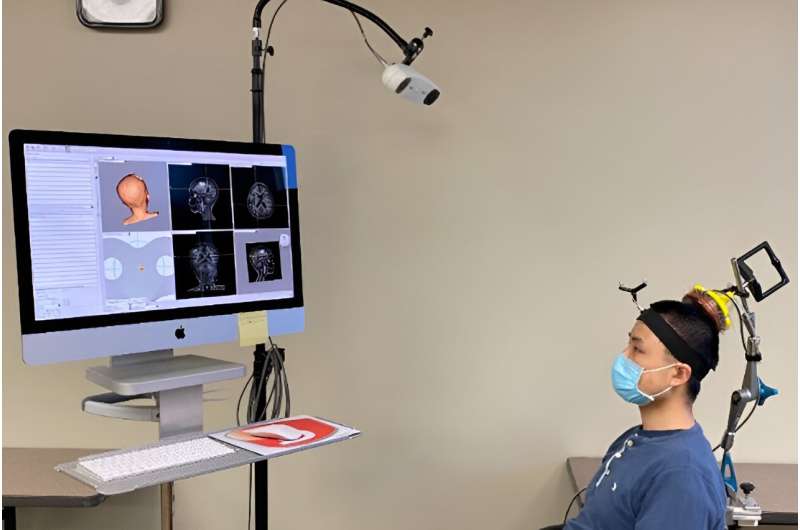
Up to now, the researchers have been using a commercially available ultrasound device that is integrated with an MRI scanner. The setup is expensive and limited to spaces with access to MRI scanning. To simplify the procedure, Chen’s team created a portable, handheld ultrasound probe and attached it to a stereotactic pointer routinely used by neurosurgeons to pinpoint brain lesions. The device was integrated into the clinical workflow without requiring additional training for neurosurgeons.
“It’s very easy to use,” Leuthardt said. “We used it in the OR for this study, but it could be used in a clinic or at a patient’s hospital bedside. It’s a step toward democratizing access to advanced diagnostics. We can interrogate patients’ brains, and we don’t need a high-tech, multimillion-dollar scanner to do it.”
Using this device, the researchers performed sonobiopsies on five people with brain tumors. Then, the tumors were surgically removed in accordance with the standard of care.
Analysis of blood samples taken before and after sonication showed that the technique increased circulating tumor DNA 1.6-fold to 5.6-fold, depending on which specific kind of DNA was analyzed. Circulating tumor DNA contains vital information about the genetic alterations in a patient’s tumor that determine how aggressively the tumor should be treated. Further, there were no signs of damage to brain tissue, indicating that the procedure is safe.
Chen and Leuthardt published their first paper describing sonobiopsy in 2018, and the potential of the technique was quickly recognized.
“Already there are multiple sites evaluating sonobiopsy in clinical trials around the world,” Chen said. “In one conference I attended recently, there was a whole session dedicated to sonobiopsy. This project exemplifies team science. Since introducing the concept of sonobiopsy in 2018, up to the publication of this clinical study, it has been a joint endeavor involving several investigators.”
More information:
Jinyun Yuan et al, First-in-human prospective trial of sonobiopsy in high-grade glioma patients using neuronavigation-guided focused ultrasound, npj Precision Oncology (2023). DOI: 10.1038/s41698-023-00448-y
Journal information:
npj Precision Oncology
Source: Read Full Article