FDA Clears the Tandem Mobi Insulin Pump
The US Food and Drug Administration has cleared the Tandem Mobi insulin pump for people with diabetes aged 6 years or older.
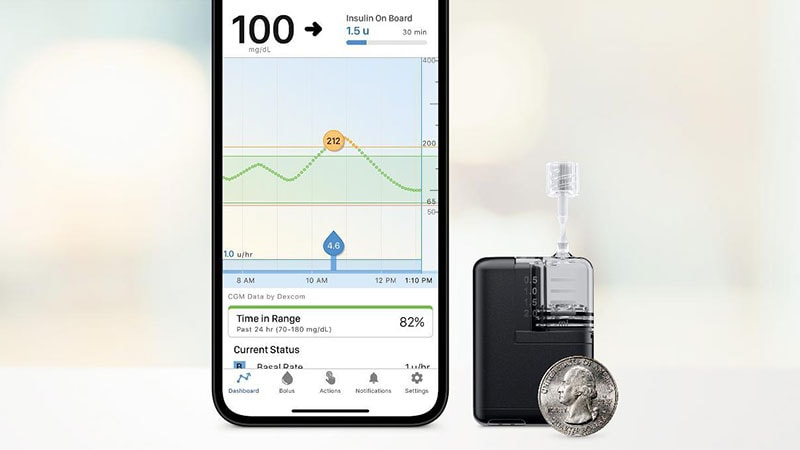
The product is half the size of the company’s t:slim X2 and is now the smallest of the commercially available durable tubed pumps. It is fully controllable from a mobile app through a user’s compatible iPhone.
Features of the Mobi include a 200-unit insulin cartridge and an on-pump button that can be used instead of the phone for bolusing insulin. The device can be clipped to clothing or worn on-body with an adhesive sleeve that is sold separately.
The Mobi is compatible with all existing Tandem-branded infusion sets manufactured by the Convatec Group, and there is a new 5-inch tubing option made just for the Tandem Mobi.
The Mobi is part of a hybrid-closed loop automated delivery system, along with the current Control-IQ technology and a compatible continuous glucose monitor (CGM). The CGM sensor predicts glucose values 30 minutes ahead and adjusts insulin delivery every 5 minutes to prevent highs and lows. Users must still manually bolus for meals. The system can deliver automatic correction boluses for up to 1 hour to prevent hyperglycemia.
Limited release of the Tandom Mobi is expected in late 2023, followed by full commercial availability in early 2024.
Miriam E. Tucker is a freelance journalist based in the Washington, DC area. She is a regular contributor to Medscape, with other work appearing in The Washington Post, NPR’s Shots blog, and Diabetes Forecast magazine. She is on Twitter @MiriamETucker.
For more diabetes and endocrinology news, follow us on Twitter and on Facebook.
Source: Read Full Article