Exploring the ‘dark side’ of Alzheimer’s disease reveals new biomarkers
Neurophysiological techniques (e.g., electroencephalography, EEG, transcranial magnetic stimulation, TMS) allow an informed investigation of the brain overexcitability underpinning the typical difficulty of maintaining a stable level of vigilance or experiencing a regular sleep-wake cycle in patients living with Alzheimer’s disease (AD). Importantly, this difficulty has a significant impact on the level of consciousness of patients, affecting patients’ ability to follow TV programs and social conversation during the daytime and preventing a deep sleep at night. As an important advantage, neurophysiological techniques can be applied in preclinical and clinical research models of the disease, its progression, and the effects of pharmacological and non-pharmacological interventions.
A special Mini-Forum published in the latest issue of the Journal of Alzheimer’s Disease reports new scientific findings and insights into the pathophysiological mechanisms that induce brain overexcitability and its deleterious clinical effects in AD patients. Such brain overexcitability can manifest as subclinical epileptiform activities often associated with a faster AD progression.
The Mini Forum has been promoted by The Electrophysiology Professional Interest Area (EPIA) Steering Committee of the International Society to Advance Alzheimer’s Research and Treatment (ISTAART). The mission of EPIA is to disseminate recent findings and concepts about the use of neurophysiological techniques (e.g., TMS and EEG) for investigating the pathophysiology inducing brain excitability at different stages of AD and the discovery of quantitative measures (biomarkers) to be used in the assessment and treatment of AD patients. The articles included in this Mini-Forum are the most recent editorial outcome of the EPIA initiative.
“These articles provide inspiring examples of preclinical and clinical research in AD using non-invasive brain stimulation and EEG techniques,” said Guest Editor Dr. Claudio Babiloni, Ph.D., professor of Physiology at Sapienza University of Rome (Italy). “The contents of those articles indicate the need to fill a gap in the actual neurobiological and clinical AD model. There is an urgent necessity for neurophysiological biomarkers to assess AD’s ‘dark side,’ whereby AD induces overexcitability in the brain neural networks with alterations in the regulation of vigilance and sleep-wake cycle. These alterations affect patients’ consciousness levels, making them unable to follow a TV talk show or a quiet social conversation among relatives, for example, negatively impacting their daily quality of life and that of their caregivers. This unique and original collection of preclinical studies in rodent models and clinical studies in AD patients covers the use of advanced neurophysiological techniques to model the pathophysiological mechanisms possibly explaining the symptoms hidden in the AD dark side.”
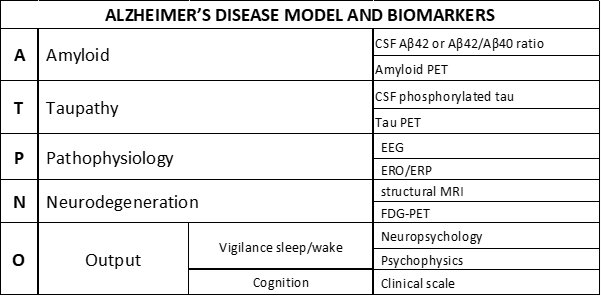
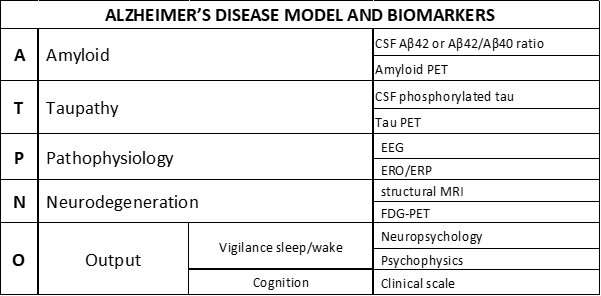
The National Institute of Aging and the Alzheimer’s Association have recently proposed a framework for the neurobiological diagnosis of AD for research applications. This ATN framework states that the AD diagnosis can be based on biomarkers derived from in vivo measurement of amyloidosis (“A”), tauopathy (“T”), and neurodegeneration (“N”) from the brain of patients living with AD. Research on cognitive deficits induced by those processes typically focuses on abnormalities in episodic memory and visuospatial skills (e.g., forgetfulness and spatial disorientation), mild cognitive impairment (MCI), and mild, moderate, or severe degrees of dementia.
The findings in this Mini-Forum provide the foundation for an extended model that could speed up research lines and discovery through further validation of pathophysiological “P” biomarkers. These are based on EEG activity/local field potentials reflecting 1) brain hyperexcitability for preclinical and clinical drug discovery pathways in AD and 2) the abnormal AD patients’ consciousness level (e.g., quiet vigilance stability) for clinical applications.
With the content of this Mini Forum in mind, Dr. Babiloni has proposed an AD model named “ATPNO,” including the symptoms and biomarkers of the AD dark side model. “Remarkably, the ATN framework may be enriched with those pathophysiological ‘P’ biomarkers and the evaluation of vigilance, sleep-wake cycle, cognitive status, and abilities in the activities of daily living as a global clinical output (‘O’) specifically,” explained Dr. Babiloni.
This Mini Forum includes six articles covering relevant areas of the pathophysiological “P” biomarkers of brain hyperexcitability in AD. They provide significant examples of the preclinical (animal studies) and clinical research in AD using non-invasive brain stimulation and EEG techniques.
Examples of the enormous potential of these new research lines in preclinical and clinical models of AD include:
In the original article, “TREM2 Deficiency Disrupts Network Oscillations Leading to Epileptic Activity and Aggravates Amyloid-β-Related Hippocampal Pathophysiology in Mice,” Milan Stoiljkovic, MD, Ph.D., Department of Comparative Medicine, Yale University School of Medicine, and colleagues tested the contribution of the so-called triggering receptor expressed on myeloid cells-2 (TREM2) of the immune cells that defend our brain, the microglia, on the control of hippocampal network hyperexcitability in transgenic mice overproducing cerebral amyloidosis typical of AD.
The investigators used an elegant combination of brain electric stimulation and the recording of local field potentials in the hippocampus in mice with transgenic mutations reducing the functioning of the microglia. Those mice showed epileptiform activity in the brain, especially when transgenic mutations also induced abnormal amyloidosis in the brain. These findings suggest that the TREM2 plays a role not only in the regulation of hippocampal neuronal excitability during physiological conditions, but also in moderating that network hyperexcitability in the case of brain amyloid-beta overproduction.
Recent evidence suggests that patients with mild to moderate AD without a known diagnosis of epilepsy might display preclinical (“silent”) epileptiform spikes during EEG recordings. These abnormal epileptiform activities occur predominantly during sleep and may be associated with accelerated disease progression. In “Alzheimer’s Disease with Epileptiform EEG Activity: Abnormal Cortical Sources of Resting State Delta Rhythms in Patients with Amnesic Mild Cognitive Impairment,” Dr. Babiloni and colleagues explored the relationship between brain network hyperexcitability and AD-related neuropathology in patients with amnesic MCI due to AD (ADMCI). None of the patients had a clinical diagnosis of epilepsy. About 15% of those patients showed silent, subclinical epileptiform EEG activity and greater AD-related amyloid neuropathology, thus suggesting more abnormal pathophysiological mechanisms underpinning the regulation of cortical arousal and quiet vigilance.
Other topics covered include:
- Functional neurophysiological biomarkers of early-stage AD
- Review of cortical overexcitability in AD and its association with clinical symptoms
- Risk stratification of cognitive decline in people with late-onset epilepsy of unknown origin
- Discussion of abnormal cortical sources of resting state delta rhythms in patients with amnesic MCI
Source: Read Full Article