Evidence-based recommendations for next-generation sequencing germline variant confirmation
The Association for Molecular Pathology (AMP) has published a report that was designed to establish recommendations for standardizing orthogonal confirmation practices for germline variants detected by next-generation sequencing (NGS).
The manuscript, “Recommendations for Next-Generation Sequencing Germline Variant Confirmation: A Joint Report of the Association for Molecular Pathology and National Society of Genetic Counselors,” was released online ahead of publication in The Journal of Molecular Diagnostics.
“While NGS has quickly transformed the field of clinical molecular genetic testing, orthogonal confirmation practices for germline variants may vary between laboratories,” said Kristy R. Crooks, Ph.D., Associate Professor and Laboratory Director at the University of Colorado Anschutz Medical Campus, and Chair of the AMP NGS Germline Variant Confirmation Working Group.
“This new report provides recommendations for orthogonal confirmation practices that, in concert with existing guidelines, are designed to help promote standardization, transparency, and quality improvement among laboratories.”
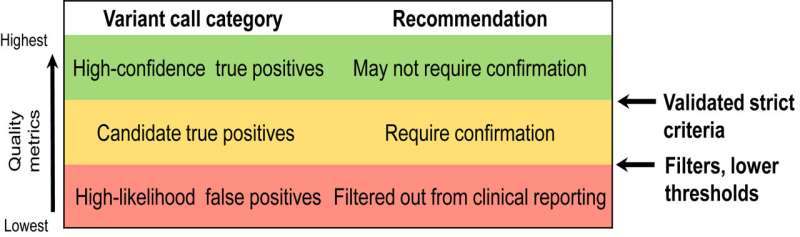
The AMP Clinical Practice Committee convened the NGS Germline Variant Confirmation Working Group to assess current evidence and standardize orthogonal confirmation practices to help limit the reporting of false positives. The new report offers the following eight recommendations that are based on a comprehensive review of published literature, empirical data, current laboratory practice surveys, feedback from open public comment, and professional experiences.
- Clinical laboratories offering germline testing using NGS should establish a written policy regarding orthogonal confirmation of NGS results.
- Laboratories’ orthogonal confirmation policy should be overseen and approved by a qualified and appropriately certified medical professional with training and experience in NGS.
- Laboratories’ confirmatory methods, platforms, and associated bioinformatics should be validated and maintained under appropriate regulatory oversight as for other aspects of the test.
- Laboratories’ confirmatory methods should be orthogonal. Discrepant results between NGS and a confirmatory assay should be investigated and resolved, rather than accepting any one methodology to be always correct.
- Laboratories should perform confirmatory testing for reported germline variants with significant clinical implications, except for variant calls meeting technical criteria rigorously demonstrated to ensure high positive predictive value from NGS alone.
- Laboratories should clearly articulate their specific policies, criteria, and methods regarding orthogonal confirmation in written materials readily available upon request.
- Laboratories’ clinical test reports should summarize orthogonal confirmation policy in every report, and when exceptions to the policy are made, these should be clearly indicated.
- Special considerations apply to certain NGS-based test types and findings.
“This new report was meant to summarize the current collective state of knowledge and guide clinical laboratory professionals regarding orthogonal confirmation of germline variants,” said Susan Hsiao, MD, Ph.D., Associate Professor of Pathology and Cell Biology at Columbia University Vagelos College of Physicians and Surgeons, and 2023 AMP Clinical Practice Committee Chair. “AMP will continue to reassess and modify our guidelines as the methodology and bioinformatics underlying NGS-based variant detection develop as part of our ongoing commitment to improving clinical practice and patient care.”
More information:
Kristy R. Crooks et al, Recommendations for Next-Generation Sequencing Germline Variant Confirmation, The Journal of Molecular Diagnostics (2023). DOI: 10.1016/j.jmoldx.2023.03.012
Journal information:
Journal of Molecular Diagnostics
Source: Read Full Article