DNA replication discovery opens paths to understanding and treating cancer, aging and degenerative disease
An international collaboration steered by David Cortez, Richard N. Armstrong, Ph.D. Chair for Innovation in Biochemistry, explored how cells tolerate DNA damage and genome instability—and they arrived at conclusions that will redirect research into DNA replication as a target for cancer and disease therapeutics.
Cells continuously divide as the body grows and renews. Before a cell divides, it copies its DNA so that both resulting cells have their own complete genome. The area along the DNA’s double strand where helix is unwound to replicate the genetic sequence is called the replication fork.
Occasionally, DNA replication is slowed or stalled by damage. Replication is also challenged when an oncogene—a gene that can transform a cell into a tumor cell—is activated. A common response to DNA replication stress is called “replication fork reversal.”
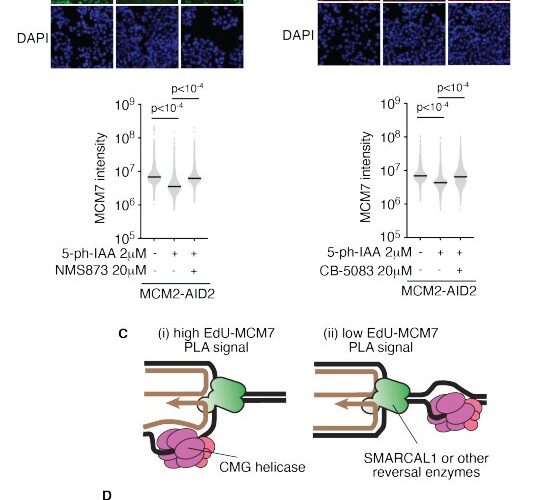
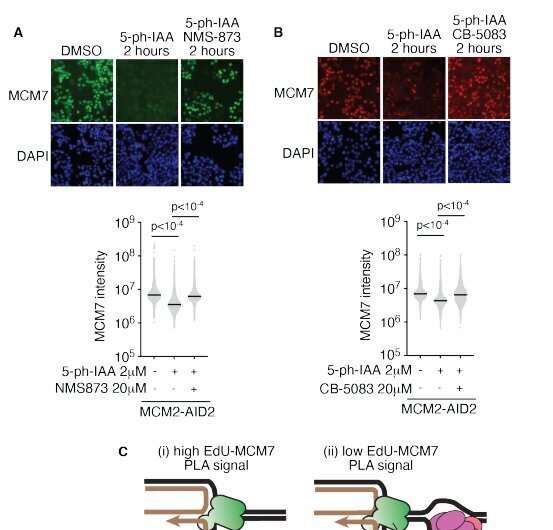
“Fork reversal is thought to be a backward movement of the fork, in which the parental DNA strands rejoin, and a new daughter strand duplex is formed,” Cortez said. “Despite years of research, how this type of DNA gymnastics happens is unclear.”
The research, led by postdoctoral fellow Wenpeng Liu, discovered a mechanism that allows cells to accomplish healthy DNA reversal while tolerating replication stress. The mechanism, dependent on the protein RAD51, answers long-standing questions in the field and completely changes the fork reversal model.
This new model says that the DNA “fork” that is reversed isn’t even the replication fork generated by the enzyme that unwinds the double-stranded DNA. “Our conclusions explain how cells can copy their DNA during every cell division cycle without generating errors that lead to diseases like cancer,” Cortez said.
Other collaborators on the paradigm-shifting research included Alessandro Vindigni at Washington University in St. Louis and Masato Kanemaki at the National Institute of Genetics in Japan. The study is published in the journal Science.
Understanding how cells copy genomes and cope with DNA damage is vital to understanding life because DNA replication is a constant process. In humans, DNA replication damaged by stress causes inaccurate gene expression, which leads to defects that contribute to cancer, degenerative disease and the effects of aging.
A fundamental understanding of how DNA replication works is a first step to fixing abnormalities that ultimately cause disease. This is a targetable avenue of study to continuing to develop better therapies for disease.
This data answers two long-standing questions in the field, but it also raises several new ones, said Cortez, who is also associate director for basic science research at Vanderbilt-Ingram Cancer Center. His lab and collaborators will now work to understand how fork reversal prevents mutations and how it is regulated to prevent potential harmful consequences for genome stability.
More information:
Wenpeng Liu et al, RAD51 bypasses the CMG helicase to promote replication fork reversal, Science (2023). DOI: 10.1126/science.add7328
Journal information:
Science
Source: Read Full Article