Developing a 'fingerprint' test that could diagnose Alzheimer’s early
.jpg) Thought LeadersDr. Cynthia McMurraySenior ScientistLawrence Berkeley National Laboratory
Thought LeadersDr. Cynthia McMurraySenior ScientistLawrence Berkeley National LaboratoryIn this interview, we spoke to Dr. Cynthia McMurray about her latest research into Alzheimer's disease and how she has developed a 'fingerprinting' technique that may help to diagnose Alzheimer's before symptoms begin to appear.
Alzheimer’s disease has been estimated to be the 6th leading cause of death in the US and also affects millions of people worldwide each year. What inspired your latest research into Alzheimer’s disease?
Alzheimer’s is one of the major problems in the world and 2% of Americans will be affected in the next coming decade. It is only going to get exponentially worse because we have no way to detect it. That is one of the reasons I am very interested. There are also no therapeutics or ways to detect Alzheimer’s and there is no genetics that goes with it. It is a big mystery.
So, I wondered what is it that ties these people together? When we do not know anything about it, how do we approach the problem? I wanted to look at whether we could detect them by looking if they were chemically alike. Chemistry is physiology. So, if you treat someone, you are taking advantage of their chemistry. My idea was; are there chemical shared features in large populations of Alzheimer’s patients, and would they give us any indication of how they might respond to therapeutics?
What is the current course of research and treatment for this condition? Are there any limitations to this? If so, what are they?
There are no treatments for Alzheimer's. That much is simple. There have been around 150 failed clinical trials over the years. An antibody for Alzheimer’s disease has been developed recently that has been approved by the FDA, but whether it is going to be effective or not, is unclear.
One of the avenues that we think this technology will be beneficial for is therapeutics. We hope the prediction algorithm will be able to not only define patients, but help to predict therapeutic groups.
Even if we know nothing, we can use this technique to follow the course of the disease and recovery by therapeutic agents. We might be able to predict a new treatment window. At the moment, no one really knows when to start treating a patient and when to stop it. A measure of this would be very beneficial.
Currently, even if we can just identify someone as an Alzheimer's patient, that is a benefit to the patient and their family. They can plan and think about major caregivers. Patients can start going on dietary regimes that might help them. There are a lot of benefits to knowing.

Image Credit: Orawan Pattarawimonchai/Shutterstock.com
Currently, diagnosing Alzheimer’s disease at its early stages is very difficult. Why is this and what are the benefits of being able to detect this disease early?
Usually, it is because the signs that are seen are very mild. Take forgetfulness. You probably have a forgetful relative. These are just normal phenotypes of a person. It is very hard to say that it is uniquely Alzheimer's.
Another feature of Alzheimer's is the beta peptides. In normal people, two types form. One is the bad form, and one is the better form. Because they form in normal individuals as well, even beta aggregates or beta complexes cannot be used as definitive indicators. Alzheimer's takes around 11 years to start measurably expressing phenotypes. That early stage is very difficult. I do not want to say impossible, but being able to predict it would be huge.
Due to its difficulty in early detection and prediction abilities, developing therapeutics for Alzheimer’s disease is also proving challenging. How could your new research help to change this?
What we are trying to do is see if we can find patients that are chemically alike. It is the idea that every cell has a signature, and the disease has a signature. Perhaps some people are chemically alike and might form a group.
When you take something like Alzheimer's, of which you do not know the basics, you are talking about a huge population of individuals. There is no way to treat all those millions of people the same way when you do not even know what it is.
They could all have different mutations and their systems are combinatorial. The idea that somehow one size fits all becomes quite useless. That idea is useful if you have a known mutation, but with something like Alzheimer's, you are just shooting in the dark. You have to find ways to approach the problem that are different and cannot rely on more standard techniques.
One of the most common solutions is biomarker development. Biomarkers are what everybody hopes to have – a molecule that changes with the disease and can be followed. That has not been particularly successful in diseases where you do not know the origins of the disease. The biomarker could be different from person to person, and often this method is not useful.
Our method gives you a signature feature of you or your disease state. It is not a biomarker. Fourier transform infrared (FTIR) measures all the molecules in the cell. These things are so ultrasensitive, that the sum of tens of thousands of molecules in every cell gives you the signature. Every cell has some of the same chemistry, but they are not put together the same way.
So, for example, two gene products in a cell may be interacting with slightly different proteins and therefore they give a little bit of a different signal. Even though they have the same basic chemistry in their environment, they react differently.
This is an integrated technique, which is a much better method for looking at states. We are not following a molecule in that cell, we are looking at this integrated signature that tells us the average of what that cell is doing. If it is in the disease category, it is a signature of disease. It has a big benefit in that regard.
Can you describe how you carried out your latest research into spectral phenotyping and Alzheimer’s disease?
It is a computational method. We took cells and scanned them with light, irradiating them with natural light to make them excited. The cells react to the light and vibrate in a certain way. We then collected that information. Every one of the vibrations is a frequency and those different signals are all captured together.
We built the algorithm to take these signals and come out with a signature. It is a simple collection of data from a microscope, which goes over to a computer. The computer takes that information, does some manipulation and calculation, and comes up with a signal.
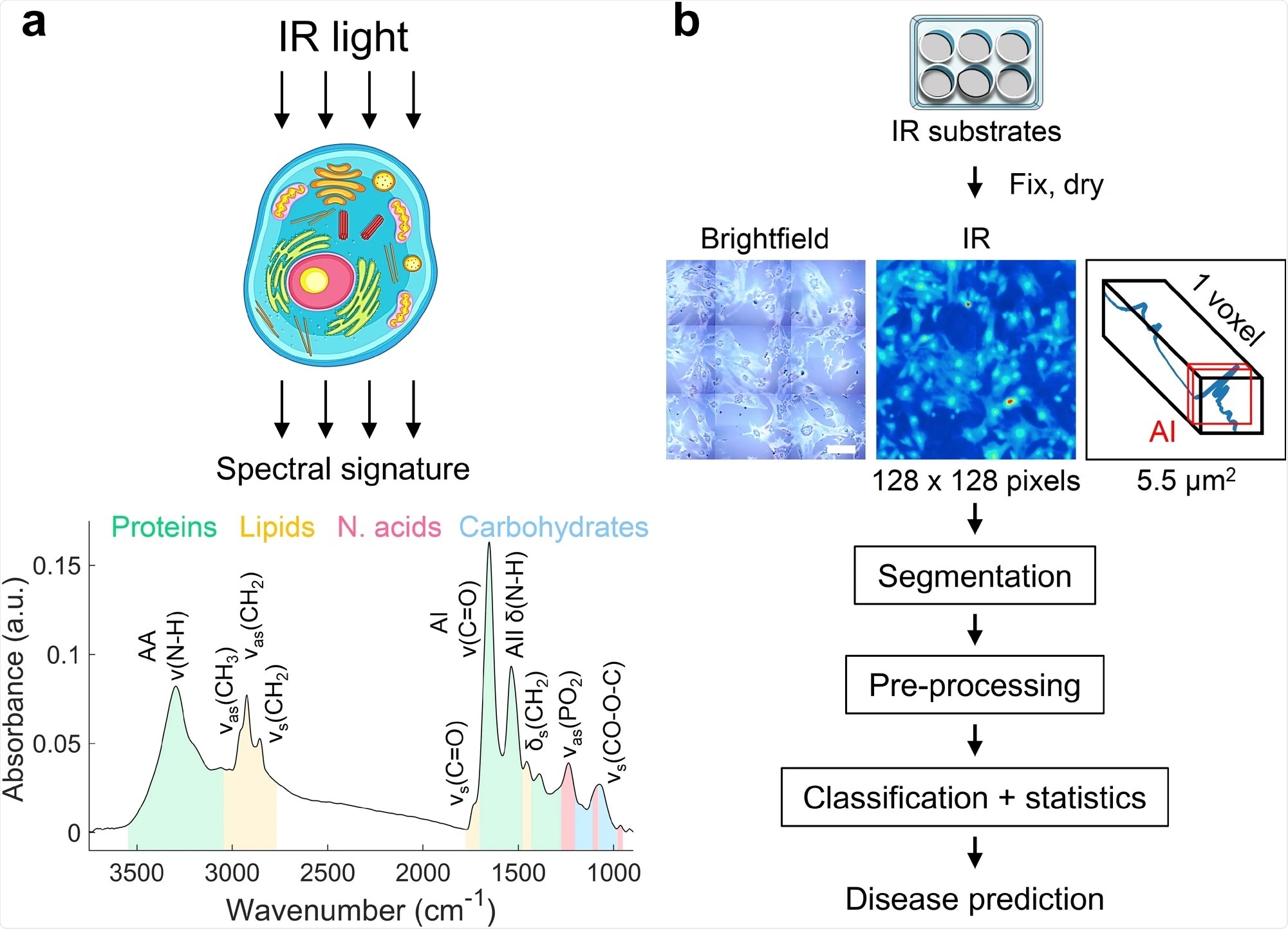
Image Credit: An infrared spectral biomarker accurately predicts neurodegenerative disease class in the absence of overt symptoms
What did you discover?
One of the real problems with neurological diseases is that no one can donate their brain when they are alive. The idea that you could use a surrogate cell that is easily accessible, was a huge benefit. We did not have to use the tissue, we only needed some skin cells from them and we could monitor what was going on in the brain.
Even though skin cells are not brain cells, they have the same genetics as the patient. They respond in their own way so they can mark the following of the disease. We are not learning anything about neuron function, but it acts as a marker and these cells can tell us about the progression of what we are looking for.
To be able to find that is a big deal. One of the often mistaken ideas about this technology is that you use it as pathology. While we could do that, this is useless at that stage. We wanted to do something very early on in a living patient so that there would be a chance to do something. This is not something that is going to take the place of pathology or anything like that. It is simply a method for determining early on whether you are in a disease group. I think these things have great power if used in large populations, which we are trying to do now.
Often biomarker-centric approaches are used when studying diseases including Huntington’s disease. What is meant by a biomarker-centric approach and why was this approach not applicable to Alzheimer’s disease?
Huntington's is probably the classic case of a single gene defect. We study Huntington's primarily for that reason. We know that there is no other cause of disease except this one thing. That is the ideal case of a biomarker. You can scan it, you know what the gene is, and you can tell it right away.
This is the case for a lot of mutation-based diseases. You can screen for the mutation, and it acts as a simple biomarker.
The second level of biomarkers is not just having a gene, but something that you look at over the course of a patient’s lifetime. For example, in cancers, a lot of times you can pick up on a couple of genes that seem to come up and down with the cancers. Those signals are followed. Even though they are not the actual mutation, that is the most common biomarker.
However, you cannot pick a small group of genes and say you know the disease. Those genes are never unique to the disease. They may be indicators, and I think that that is very powerful, but they are not good enough. You might recognize that this is a gene that has been seen in other cancers, but that does not mean much in the long run.
For example, if you can measure all 13,000 genes and all the splice products and all the things that people try to do with transcriptomes, theoretically you might be able to find something. But in reality, you never can. All kinds of physiology go into gene expression and it is not necessarily all captured in finding a few genes that seem to be biomarkers.
Our method can occur early and provide a fingerprint totally unique to those cells and diseases. It is telling you about the state of something. We can do this for cancers or any disease, not just Alzheimer’s. We have focused on the brain because I am interested in that, and it is the most difficult area.
How will your research not only help progress research efforts and new treatments but also improve the quality of life for patients suffering from the disease?
If you have an early diagnosis, you have the life of the patient to figure out an option. Right now, there are no therapeutics and so we do not have that to rely on. If we know that you are an Alzheimer's patient before you really start developing severe symptoms, then you can plan and your family can plan.
Alzheimer's can be influenced by diet and lifestyle. The onset of the disease and its severity can sometimes be modulated slightly. There are a lot of benefits to the families who bear the burden, as it enables them to structure their lives or their finances. And for the patient, there may be a treatment down the road, in which case it would direct the patient to therapeutics.
Infrared spectroscopy (IR) is used in a variety of scientific fields including agriculture, material sciences, and earth sciences. However, it is rather unpopular within the medical science community. Why is this and do you hope that your research will highlight the many uses of IR, potentially encouraging other people to use it also?
There are two reasons why. First, it has been used in agriculture and other areas because nothing gets changed. It is a detection method and it works fairly well. In the medical sector, a clinician has to use it to do a diagnosis. That is a learning curve. Clinicians need to, as a group, feel they can count on this as a technique.
The second is that with the complexity of humans, it was not necessarily thought that IR could pick up these kinds of differences. We certainly have to ask ourselves the question because we have used proof of principle experiments when we go to thousands of patients. Right now, it seems like no matter how much we are amplifying the group's number, we are still able to see this. We are going to need large populations. There is this idea that complexity of the humans may interfere with this.
One of the other issues that people question is how you can use this method for every disease. But vibrational units are so sensitive that they come up with their own uniqueness. Another question is, can you tell every disease apart from each other? For every new disease that we do, we always plot them on the same plot. Right now we have a beautiful separation, without any overlap in the diseases themselves. They all form their own group. We have no idea until we collect data what is going to happen for all diseases or even all neurological diseases.
What further research needs to be carried out before this technique can be applied within a medical setting?
We came up with a computational method to do this. We were not taking physiological tests or anything about these patients. We did not have to know anything about the sample. That is a benefit of this approach.
We built this algorithm to do this and there are ways to improve the algorithm. That is what we are doing now, trying to go through every part of how we do the calculation and see if we can do it better and refine the methods.
We are also trying to do some visualization. The technique itself is not limited to medical applications at all. One of the things that we are looking for, for example, is slicing the brain. Say we want to know if something has a disease, what is in the disease, and what is being affected. We can go, for example, into a mouse and cut up their brains. We can scan the whole brain and see the chemical changes in all the cells. We can identify what is going on, for example, we could identify that a particular group of cells is changing. Then we could pull those cells out and do some more sophisticated tests. This is a really good way to try to watch things.
In neurological disorders, you only get the brains of people at the end of their life. It turns out that if you are looking at the beginning, you might find that the regions of the brain affected at 90 or 80 are not at all where it started. It might have started in a whole different place and may change over time. We are hoping to learn more about the biology of these diseases by doing these other kinds of techniques.
Image Credit: BlurryMe/Shutterstock.com
Do you believe that with continued research into spectral phenotyping it could potentially be applied to other mystery diseases?
It can be applied to any disease. The question becomes, how does it discriminate among them? I think there is no doubt all of these things have signatures.
Say something brand new popped up in somebody. We could tell if it fell into a category for a disease, and if it was different from control. We look to see who is like that beginning and use that as our guidepost. If we had one patient that we know was affected, and a control to compare to, we could tell the patient if they fell into a group without knowing what the disease was.
In terms of known diseases, I think we are pretty capable of doing almost anything. One of the diseases I'm really interested in doing is schizophrenia. I would like to look at the kinds of things that are difficult to diagnose. I think that is where this technique is going to have real value. Nobody knows if you are going to get schizophrenia, you develop it over time until it becomes an evident problem. An early diagnosis for this would be incredible.
In a world where a lot of current scientific and medical attention is being given to COVID-19, why is it still equally as important to raise awareness for other diseases such as Alzheimer’s?
I do not think COVID is taking away from the awareness of Alzheimer's. I think Alzheimer's is here to stay – it is the biggest health problem in the US and it even exceeds COVID-19. COVID-19 is critical now because it is transmissible. It does not care about who you are, it just gets you. But there are more Alzheimer's patients than there are COVID.
COVID is a crisis, an acute problem that we have to deal with now. Alzheimer's is more of a chronic problem that goes on all the time. The number of Alzheimer's patients is so huge worldwide, that I do not think COVID would suddenly obscure all interest in Alzheimer's. It is just a very different application and it is something that we need to do now for people.
What are the next steps for you and your research?
What we are trying to do next is make this technology a solid test. My vision for the future is to have a usable biological test that clinicians want to use.
I would like to see these tests done for every patient, and for them to be recorded in a clinical record.
There are all kinds of cases where patients go to physicians and say, "Well, I'm having these symptoms, maybe I'm this." This way, all this information is there to go back and look at. If it is established, therapeutics are going to be really important.
Where can readers find more information?
- https://newscenter.lbl.gov/2021/10/06/skin-cell-alzheimers-test/
About Cynthia McMurray
Cynthia McMurray studies the biological progression of neurodegenerative diseases, such as Huntington Disease and Alzheimer's Disease, by investigating changes in brain metabolism, DNA damage, and other cellular indicators..jpg)
Posted in: Thought Leaders | Medical Science News | Medical Research News | Medical Condition News | Disease/Infection News | Healthcare News
Tags: Agriculture, Antibody, B Cell, Biomarker, Brain, Cell, Chronic, Diet, DNA, DNA Damage, Frequency, Gene, Gene Expression, Genes, Genetics, Huntington’s Disease, Metabolism, Microscope, Molecule, Mutation, Neurodegenerative Diseases, Neuron, Pathology, Peptides, Physiology, Research, Schizophrenia, Skin, Skin Cells, Spectroscopy, Therapeutics
.png)
Written by
Emily Henderson
During her time at AZoNetwork, Emily has interviewed over 200 leading experts in all areas of science and healthcare including the World Health Organization and the United Nations. She loves being at the forefront of exciting new research and sharing science stories with thought leaders all over the world.
Source: Read Full Article
