Detection of a coronavirus similar to SARS-CoV-2 in Cambodian bats
We know little about the origin and reservoir of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is responsible for the ongoing coronavirus disease 2019 pandemic (COVID-19). However, horseshoe bats tested in Yunnan province, China, have been found to carry the closest relatives of SARS-CoV-2.
Now, a new study published in the journal Nature Communications reports the identification of SARS-CoV-2 related coronaviruses in two horseshoe bats sampled in Cambodia in 2010.

Coronaviruses and bats
The origin, reservoir, diversity, and extent of circulation of SARS-CoV-2 ancestors are unknown; however, Horseshoe bats are believed to be the primary natural reservoirs for SARS-related coronaviruses. Horseshoe bats from several provinces in China have been found to carry several coronavirus species. Southeast Asia is home to more than 25% of the world's bat species and is considered a hotspot for emerging zoonotic diseases. A close relative of SARS-CoV-2 was identified in bats captured from a cave in Thailand in June 2020.
Cambodia
UNESCO and the National Authority of Preah Vihear mandated the Muséum national d'Histoire naturelle (MNHN, Paris, France) to conduct a mammal survey in northern Cambodia in 2010. During this survey, bats were captured using mist nets and harp traps from two provinces, Preah Vihear and Ratanakiri. Bat diversity on the two sides of the Mekong River was compared.
Likewise, USAID funded the PREDICT project to sample bats to detect and discover viruses with a pandemic and zoonotic potential. Oral and rectal swabs were collected from bats from 2012 to 2018.
The samples were tested for SARS-CoV-2 related viruses. Two bats positive for viruses closely related to SARS-CoV-2 were collected during the MNHN mission.
The investigators tested 430 archived samples, including 162 oral swabs and 268 rectal swabs. Sixteen rectal swabs (3.72%) tested positive for coronaviruses – 11 alphacoronaviruses and five betacoronaviruses. Two of the five betacoronavirus samples further tested positive using a specific reverse transcriptase quantitative polymerase chain reaction (RT-qPCR) test. These two samples were from rectal swabs of Shamel's horseshoe bats sampled in December 2010 in the Steung Treng province in Cambodia. Oral swabs from these bats tested negative for betacoronaviruses.
Genetic similarity with SARS-CoV-2
The RNA from the two coronaviruses was then processed for next-generation metagenomic sequencing. The sequenced genome was compared to the SARS-CoV-2 genome.
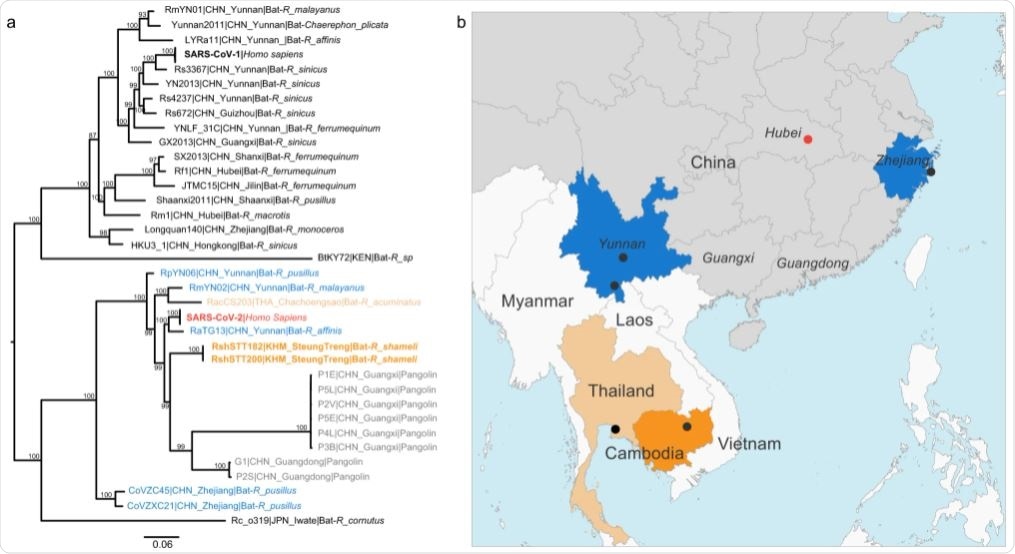
The two genomic sequences were closely related to SARS-CoV-2, with 92.6% identity across the genome. They also exhibited identical genomic organization. Thus, these two coronaviruses are a sublineage of SARS-CoV-2 related viruses, even though they are geographically distant.
A portion corresponding to the N terminal domain of the spike protein was dissimilar with SARS-CoV-2. This region was similar to more distantly related betacoronaviruses.
This data suggests that the ancestors of these viral sublineages co-circulate within a wider geographic area and more distinct bat species.
Receptor-binding domain
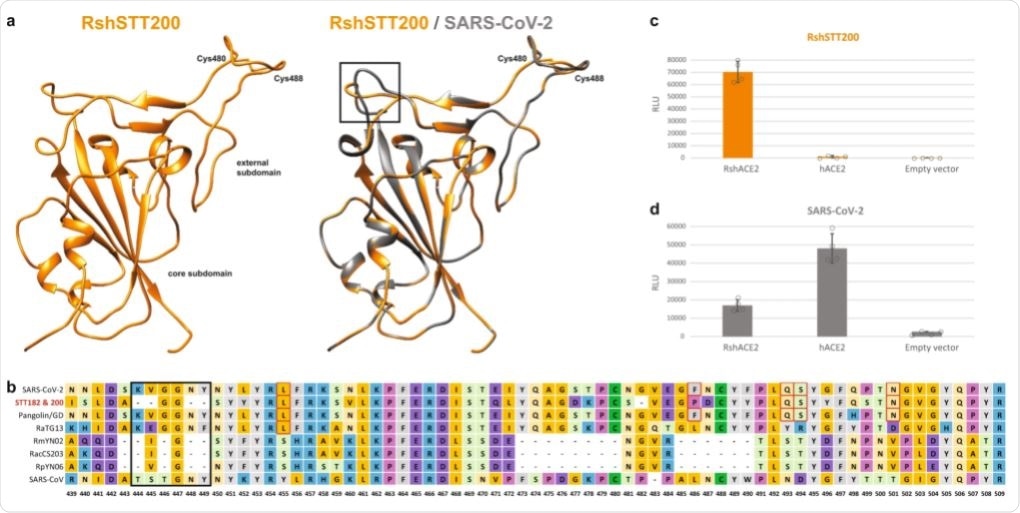
In silico structural analysis suggested that the external subdomain of the spike receptor-binding domain (RBD) structure is highly similar to SARS-CoV-2. Six amino acid residues determine efficient receptor binding of SARS-CoV-2 to the human angiotensin-converting enzyme 2 (hACE2) receptor. Five out of six amino acid residues are conserved.
The investigators then performed pseudovirus entry assays using HEK293T cells expressing either the bat ACE2 receptor or human ACE2 receptor. Viral pseudoparticles packaged a coronavirus spike from SARS-CoV-2 or the bat coronavirus. Pseudoviral particles expressing the bat coronavirus spike were not able to infect HEK293T cells expressing human ACE2 but they were able to infect HEK293T expressing bat ACE2. In addition, the pseudoviral particles expressing the SARS-CoV-2 spike were able to infect HEK293T cells expressing bat ACE2.
Conclusions
This study discovered SARS-CoV-2 related viruses in a bat species not found in China. This implies that these viruses have a much wider geographic distribution than previously reported. This study also suggests that Southeast Asia should be considered for future surveillance for coronaviruses.
Southeast Asia has a high diversity of wildlife. There also exists extensive trade in wild animals. Moreover, there is a dramatic land-use change due to infrastructure development, urban development, agricultural expansion, and human encroachment upon wildlife. Due to these factors, human contact with wild hosts of SARS-like coronaviruses has increased. Therefore, Southeast Asia may represent an area to consider for the ongoing research for the origins of SARS-CoV-2.
This study also suggests that Southeast Asia should be considered for future surveillance for coronaviruses.
According to this study, continued and expanded surveillance of bats and other wild animals in Southeast Asia is crucial for future pandemic preparedness and prevention.
- Delaune, D., Hul, V., Karlsson, E.A. et al. (2021) A novel SARS-CoV-2 related coronavirus in bats from Cambodia. Nature Communications 12, 6563. https://doi.org/10.1038/s41467-021-26809-4
Posted in: Medical Science News | Medical Research News | Disease/Infection News
Tags: ACE2, Amino Acid, Angiotensin, Angiotensin-Converting Enzyme 2, Coronavirus, Enzyme, Genetic, Genome, Genomic, Pandemic, Phylogeny, Polymerase, Polymerase Chain Reaction, Protein, Pseudovirus, Receptor, Research, Respiratory, Reverse Transcriptase, RNA, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome

Written by
Dr. Shital Sarah Ahaley
Dr. Shital Sarah Ahaley is a medical writer. She completed her Bachelor's and Master's degree in Microbiology at the University of Pune. She then completed her Ph.D. at the Indian Institute of Science, Bengaluru where she studied muscle development and muscle diseases. After her Ph.D., she worked at the Indian Institute of Science, Education, and Research, Pune as a post-doctoral fellow. She then acquired and executed an independent grant from the DBT-Wellcome Trust India Alliance as an Early Career Fellow. Her work focused on RNA binding proteins and Hedgehog signaling.
Source: Read Full Article
