Sinovac COVID-19 vaccine shows 50% effectiveness in a cohort of Brazilian healthcare workers
CoronaVac is a well-tolerated vaccine containing inactivated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and it induced humoral responses in phase 1 and 2 studies. Analysis of a Brazilian phase 3 clinical trial that evaluated 12,607 participants showed that the vaccine had an efficacy of 50.39% 15 days after the 2nd dose.
Vaccine efficacy refers to the proportion of reduction in disease among vaccinated individuals in a clinical trial, while vaccine effectiveness is the reduction in disease vaccinated persons in real-world conditions. Studies have not focused on the effectiveness of CoronaVac so far.
“Despite the studies of the efficacy of vaccines against COVID-19, little is known about their effectiveness, or how they perform in real-life conditions.”
Estimating the effectiveness of the CoronaVac vaccine in healthcare workers in Brazil
Researchers from Brazil recently reported the occurrence of symptomatic COVID-19 in a group of healthcare workers vaccinated with CoronaVac. They also estimated the effectiveness of the vaccine. The study is published on the preprint server, medRxiv*.
CoronaVac was given to the study cohort of 22,402 healthcare workers in Hospital das Clinicas during 18 and 21 January 2021 (epi week 3) and 21,652 healthcare workers during 14-16 February 2021 (epi week 7).
“A Poisson regression model was used to study the relationship between the weekly number of COVID-19 cases among HC HCW, taken as a dependent variable, and cases recorded for the general population of São Paulo city.”
Cases of symptomatic COVID-19 were evaluated weekly. In epidemic (epi) weeks 3-12 of 2021, the predicted numbers of cases among healthcare workers were compared to the actual numbers of cases post-vaccination. For weeks 9-12, 2 to 5 weeks after the 2nd dose of the vaccine, effectiveness was estimated. A total of 142 samples from vaccinated individuals were tested for SARS-CoV-2 variants of concern.
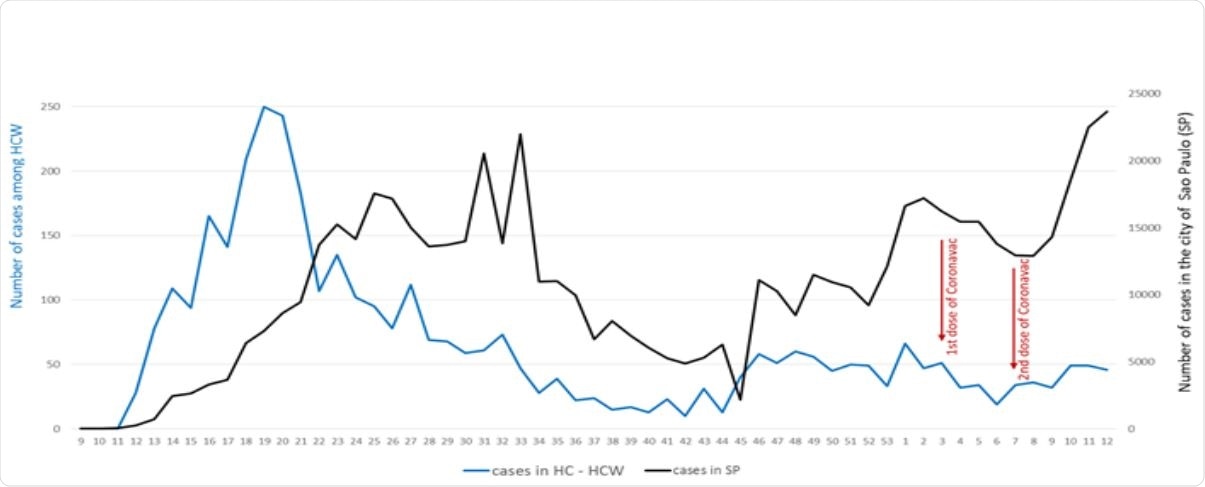
CoronaVac was found to have ~50% effectiveness 2 and 3 weeks after the second dose and the effectiveness increased over time
A total of 380 healthcare workers were diagnosed with COVID-19 since the first dose of the vaccine. The number of COVID-19 cases in the city increased sharply in 2021, but the number of cases among the healthcare workers did not follow the same trend.
The estimated effectiveness of the vaccine 2 and 3 weeks after the 2nd dose was 50.7% and 51.8%, respectively. Effectiveness increased over the next 2 weeks. Forty-seven percent of the samples, or 67 out of 142, had variants of concern, mostly P1.
“The estimated effectiveness 2 and 3 weeks after 2nd dose was 50.7% and 51.8%, respectively, and increased over the next two weeks. 67/142 samples (47%) were variants of concern, mostly P1.”
Findings reflect the variant distribution in the community
The prevalence of variants in nearly 50% of the studied samples is interesting. There have been concerns about vaccination leading to an increase in infections by variants as vaccines may not be efficient against newer variants of the virus. However, the authors believe that the high prevalence of P1 and other variants reflects the epidemiological situation in the region.
In November 2020, the P1 variant was said to be emerging in the Amazon region in Brazil. It spread rapidly and became the predominant strain within 7 weeks, accounting for 87% of COVID-19 cases and dominating the cases in several regions of Brazil. The city of São Paulo recently reported 64% of P1 and 7% of B.1.1.7 variants in samples collected between 16 February and 6 March 2021. Thus, the authors conclude that their results reflect the variant distribution in the community.
To summarize, since the administration of the first dose of CoronaVac to the healthcare workers of the hospital, COVID-19 cases have been reduced among the healthcare workers.
“São Paulo started the second wave of COVID-19 soon after the completion of vaccination in HC, that was not reflected among our HCWs.”
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Performance of vaccination with CoronaVac in a cohort of healthcare workers (HCW) – preliminary report, Elizabeth de Faria, Ana Rubia Guedes, Maura S. Oliveira, Moacyr Vergara de Godoy Moreira, Fernando Liebhart Maia, Antonio dos Santos Barboza, Mariana Deckers Leme, Leila S. Harima Letaif, Anna Miethke-Morais, Eloisa Bonfá, Aluisio C. Segurado, Francis M. Tomazini, Alcir Alves dos Santos Jr, Pedro Figueiredo, Pâmela dos Santos Andrade, Franciane Mendes de Oliveira, Raissa Heloisa de Araújo Eliodoro, Jaqueline Goes de Jesus, Carolina dos Santos Lazari, Ester C. Sabino, Silvia F. Costa, Antonio Carlos Pedroso de Lima, Anna S. Levin, medRxiv, 2021.04.12.21255308; doi: https://doi.org/10.1101/2021.04.12.21255308, https://www.medrxiv.org/content/10.1101/2021.04.12.21255308v1
Posted in: Drug Trial News | Medical Research News | Disease/Infection News
Tags: Clinical Trial, Coronavirus, Coronavirus Disease COVID-19, Efficacy, Healthcare, Hospital, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Vaccine, Virus

Written by
Susha Cheriyedath
Susha has a Bachelor of Science (B.Sc.) degree in Chemistry and Master of Science (M.Sc) degree in Biochemistry from the University of Calicut, India. She always had a keen interest in medical and health science. As part of her masters degree, she specialized in Biochemistry, with an emphasis on Microbiology, Physiology, Biotechnology, and Nutrition. In her spare time, she loves to cook up a storm in the kitchen with her super-messy baking experiments.
Source: Read Full Article
