Mix and match COVID-19 vaccines safe and effective: German study
A new preprint study posted to the medRxiv* server shows an AstraZeneca’s ChAdOx1-nCov-19 and Pfizer/BioNTech BNT162b2 COVID-19 vaccine combination is immunologically superior to two AstraZeneca ChAdOx1-nCov-19 doses.
Led by German researchers, the study shows that having the first dose with AstraZeneca’s ChAdOx1 and the second with Pfizer/BioNTech BNT162b2 elicits significantly higher anti-spike titers and more neutralizing activity against B.1.1.7, P.1, and B.1.351 variants.
Both P.1 and B.1.351 variants have mutations that allow them to evade vaccine-induced immunity — increasing the risk of spreading SARS-CoV-2 and developing severe illness or death. In response, there have been discussions amongst vaccine developers for creating booster shots against variants.
The findings suggest mixing and matching vaccine doses could be enough to boost the immune system against different variants of concern.
Immune response from the first dose wanes over time
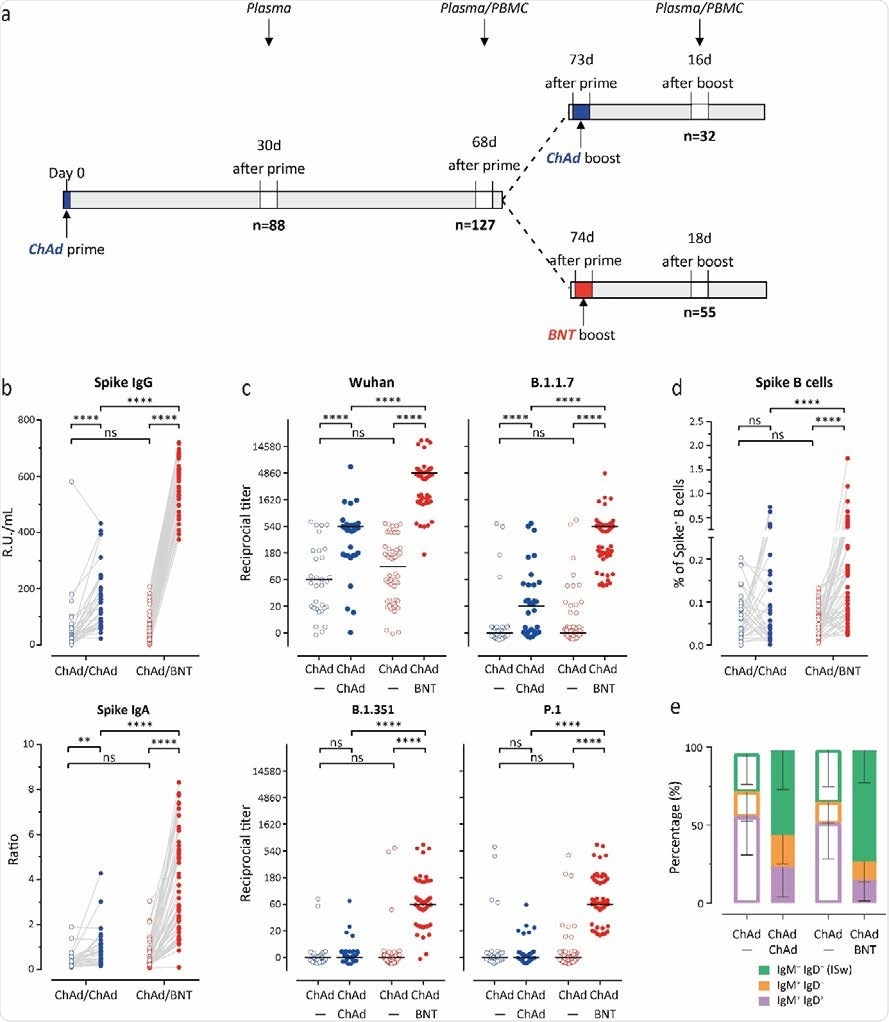
The researchers used the COVID-19 Contact Study cohort of Healthcare Professionals to evaluate immune responses from the first dose of the AstraZeneca vaccine before and 3 weeks after choosing a second dose with either the same vaccine or the Pfizer/BioNTech dose.
About 129 people who received the first AstraZeneca dose were never infected with COVID-19. Of this group, 32 chose the same booster, and 55 chose the Pfizer/BioNTech booster.
Both groups had comparable anti-S IgG and IgA antibody levels against the spike protein before receiving their second dose. However, the boosted antibody levels from the first dose declined by almost half 30 days after receiving the first dose but before getting the second.
Greater antibody response with AstraZeneca/Pfizer/BioNTech combo
Regardless of the vaccine, both groups who received a second dose showed a boost in anti-S IgG and IgA responses.
Having the Pfizer/BioNTech vaccine as the second dose significantly increased anti-S IgG antibody levels by 11.5-fold compared to a 2.9-fold increase observed in people who received both AstraZeneca vaccines. Similar increases in anti-S IgA antibody levels were also observed, suggesting a better humoral immune response with mixed-match doses.
Increased neutralizing activity against SARS-CoV-2 variants
The researchers next looked at neutralization effectiveness against SARS-CoV-2 and recent variants of concern, including B.1.1.7, P.1, and B.1.351 variants.
Of 81 out of 88 participants, neutralizing antibodies against the Wuhan strain were found. However, there were fewer neutralizing antibodies for B.1.1.7, 7 (17/88), P.1 (12/88), and B.1.351 (5/88).
Two to 3 weeks after the second vaccine dose, the researchers observed increases in titers of neutralizing antibodies against the Wuhan strain in both groups — with the mix-match group showing the highest neutralizing titers.
Against the variants of concern, getting two doses of AstraZeneca showed some immune response towards the B.1.17 variant. However, it was not effective against P.1 or B.1.351.
Getting a second Pfizer/BioNTech dose increased neutralizing antibodies against all variants of concern. The AstraZeneca- Pfizer/BioNTech group had the highest neutralizing response against the Wuhan strain, followed by the B.1.1.7 variant. While some neutralization was observed, the mixed-match doses were less efficient against the P.1 and B.1.351 variants.
“Altogether, these data indicate that the booster immunization led to an increase of neutralizing antibodies in both vaccination groups and that the heterologous ChAdOx/BNT booster booster vaccination efficiently induced neutralizing antibodies against all tested VoC.”
Higher number of B cells in Pfizer/BioNTech combo group
S-specific B cells strongly increased, and an increase in recent isotype switched B cells (IgD–IgM–) was observed in people who received Pfizer/BioNTech as a second dose.
The increase in B cells specific to the spike protein after receiving the Pfizer/BioNTech booster corresponded with neutralizing power against the variants of concern.
There were also greater increases in S-specific CD4 T cells and S-specific IFN-γ release in the mixed-matched group.
Future work
The results suggest mixing booster shots elicits a more significant immune response against SARS-CoV-2. However, more work is needed to characterize immune responses further. It would also help to understand how long vaccine immunity lasts and whether mixed-match doses provide a more robust immune response over time.
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Barros-Martins J, et al. Humoral and cellular immune response against SARS-CoV-2 variants following heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. medRxiv, 2021. doi: https://doi.org/10.1101/2021.06.01.21258172, https://www.medrxiv.org/content/10.1101/2021.06.01.21258172v1
Posted in: Drug Trial News | Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: Antibodies, Antibody, CD4, Coronavirus Disease COVID-19, Healthcare, Homologous, Immune Response, Immune System, Immunization, Protein, SARS, SARS-CoV-2, Spike Protein, Vaccine

Written by
Jocelyn Solis-Moreira
Jocelyn Solis-Moreira graduated with a Bachelor's in Integrative Neuroscience, where she then pursued graduate research looking at the long-term effects of adolescent binge drinking on the brain's neurochemistry in adulthood.
Source: Read Full Article
