GluN3A knockout mouse: Alternative model for Alzheimer’s neurodegeneration
In recent debate over the FDA’s approval of the Alzheimer’s drug aducanumab, we’ve heard a lot about the “amyloid hypothesis.” In that context, it’s refreshing to learn about a model of Alzheimer’s neurodegeneration that doesn’t start with the pathogenic proteins amyloid or Tau.
Instead, a new paper in Alzheimer’s & Dementia from Emory neuroscientist Shan Ping Yu and colleagues focuses on an unusual member of the family of NMDA receptors, signaling molecules that are critical for learning and memory. Their findings contain leads for additional research on Alzheimer’s, including drugs that are already FDA-approved that could be used preventively, and genes to look at for risk factors.
“It’s not just another rodent model of Alzheimer’s,” Yu says. “We are emphasizing a different set of mechanisms leading to neurodegeneration.”
Those mechanisms include alterations in calcium and neuronal hyperactivity, which occur first in this mouse model, rather than standard models that have clumps of amyloid or Tau as the primary drivers.
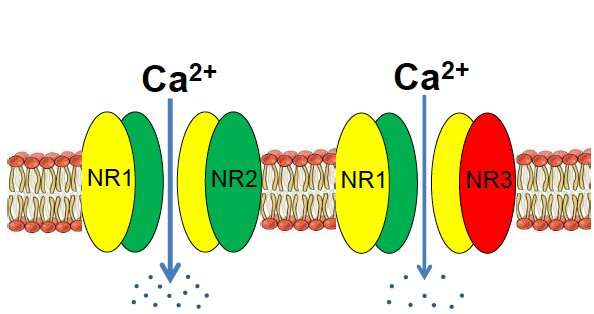
For the last several years, Yu and his laboratory have been studying the NMDA receptor subunit GluN3A in the context of stroke and also brain development. According to their research, GluN3A acts like a control rod in a nuclear reactor, cooling down signaling in the brain so that things don’t overheat. It’s an inhibitory part of a receptor assembly that is usually stimulatory.
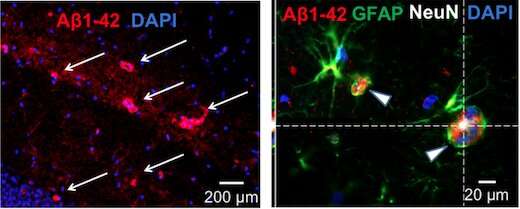
Yu says GluN3A’s role in the adult brain is understudied, because it is generally thought to fade away after early development. Mice that are missing the gene for GluN3A get a benefit earlier in life, in that they have enhanced memory and spatial learning. But later on, the missing gene’s function catches up, and the mice develop several features of Alzheimer’s, including olfactory deficits, cognitive decline, neurodegeneration and neuroinflammation, and eventually amyloid/tau pathology.
“We show that virtually all clinical symptoms and pathophysiology spontaneously developed in the GluN3A knockout mouse in an age-dependent manner,” Yu says.
Yu says he was originally motivated to examine GluN3A’s role in neurodegeneration because the GluN3A-knockout mice develop the early symptom of olfactory dysfunction, which is commonly seen in Alzheimer’s and Parkinson’s patients. In the current paper, Yu and colleagues show that loss of GluN3A leads to elevated calcium levels, normally tightly regulated, and what they call “degenerative excitotoxicity”.
This is distinct from the excitotoxicity that is harmful in traumatic brain injury or stroke—milder and more chronic. They connect the hyperactivity and inflammation to the “calcium hypothesis” for Alzheimer’s—a well-established idea that dysregulated calcium drives neurodegeneration. Yu says that their discovery of the role of GluN3A relates more to the early stages of the disease, before amyloid plaque formation.
Source: Read Full Article
