ECDC Gives Guidance on Prevention and Treatment of Monkeypox
In a new risk-assessment document, the European Centre for Disease Prevention and Control (ECDC) summarizes what we currently know about monkeypox and recommends that European countries focus on the identification and management of the disease as well as contract tracing and prompt reporting of new cases of the virus.
Recent Developments
From May 15 to May 23, in eight European Union (EU) member states (Belgium, France, Germany, Italy, the Netherlands, Portugal, Spain, and Sweden) a total of 85 cases of monkeypox were reported; they were acquired through autochthonous transmission. Current diagnosed cases of monkeypox have mainly been recorded in men who have sexual relations with other men, suggesting that transmission may occur during sexual intercourse, through infectious material coming into contact with mucosa or damaged skin, or via large respiratory droplets during prolonged face-to-face contact.
Andrea Ammon, MD, director of the ECDC, stated that “most current cases have presented with mild symptoms of the disease, and for the general population, the chance of diffusion is very low. However, the likelihood of a further spread of the virus through close contact, for example during sexual activities among people with multiple sexual partners, is considerably increased.”
Stella Kyriakides, European commissioner for health and food safety, added, “I am worried about the increase of cases of monkeypox in the EU and worldwide. We are currently monitoring the situation and, although, at the moment, the probability of it spreading to the general population is low, the situation is evolving. We should all remain alert, making sure that contact tracing and a sufficient diagnostic capacity are in place and guarantee that vaccines and antiviral drugs are available, as well as sufficient personal protective equipment [PPE] for healthcare professionals.”
Routes of Transmission
Monkeypox is not easily spread among people. Person-to-person transmission occurs through close contact with infectious material, coming from skin lesions of an infected person, through air droplets in the case of prolonged face-to-face contact, and through fomites. So far, diagnosed cases suggest that transmission can occur through sexual intercourse.
The incubation period is 5-21 days, and patients are symptomatic for 2-4 weeks.
According to the ECDC, the likelihood of this infection spreading is increased among people who have more than one sexual partner. Although most current cases present with mild symptoms, monkeypox can cause severe disease in some groups (such as young children, pregnant women, and immunosuppressed people). However, the probability of severe disease cannot yet be estimated precisely.
The overall risk is considered moderate for people who have multiple sexual partners and low for the general population.
Clinical Course
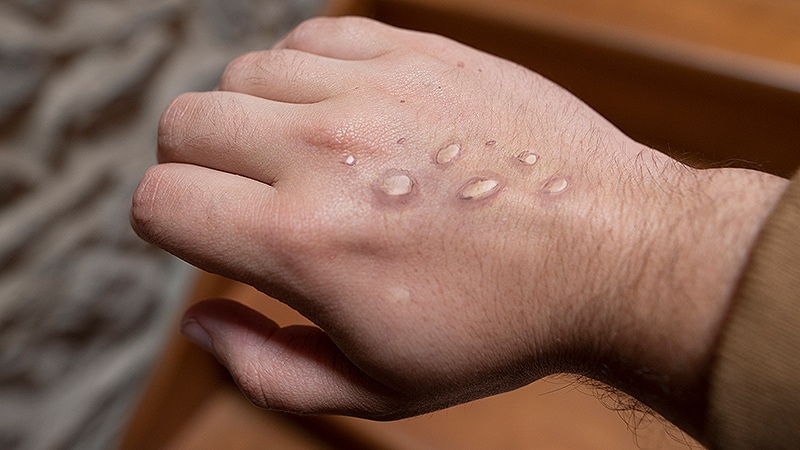
The disease initially presents with fever, myalgia, fatigue, and headache. Within 3 days of the onset of the prodromal symptoms, a centrifugal maculopapular rash appears on the site of primary infection and rapidly spreads to other parts of the body. The palms of the hands and bottoms of the feet are involved in cases where the rash has spread, which is a characteristic of the disease. Usually within 12 days, the lesions progress, simultaneously changing from macules to papules, blisters, pustules, and scabs before falling off. The lesions may have a central depression and be extremely itchy.
If the patient scratches them, a secondary bacterial infection may take hold (for which treatment with oral antihistamines is indicated). Lesions may also be present in the oral or ocular mucous membrane. Either before or at the same time as onset of the rash, patients may experience swelling of the lymph nodes, which usually is not seen with smallpox or chickenpox.
The onset of the rash is considered the start of the infectious period; however, people with prodromal symptoms may also transmit the virus.
Most cases in people present with mild or moderate symptoms. Complications seen in endemic countries include encephalitis, secondary bacterial skin infections, dehydration, conjunctivitis, keratitis, and pneumonia. The death rate ranges from 0% to 11% in endemic areas, with fatalities from the disease mostly occurring in younger children.
There is not a lot of information available on the disease in immunosuppressed individuals. In the 2017 Nigerian epidemic, patients with a concomitant HIV infection presented with more severe disease, with a greater number of skin lesions and genital ulcers, compared with HIV-negative individuals. No deaths were reported among seropositive patients. The main sequelae from the disease are usually disfiguring scars and permanent corneal lesions.
Treatment
No smallpox vaccines are authorized for use against monkeypox, however the third-generation smallpox vaccine Imvanex (Modified Vaccinia Ankara) has been authorized by the European Medicines Agency (EMA) for the EU market against smallpox and has demonstrated to provide protection in primates.
Old-generation smallpox vaccines have significant side effects, are no longer authorized, and should no longer be used. It is also important to note the lack of safety data for the use of Imvanex in immunocompromised people.
For this reason, National Immunization Technical Advisory Groups have been asked to develop specific guidelines for vaccination in close contacts of patients with monkeypox. The use of a smallpox vaccine for preexposure prophylaxis cannot be considered now, when taking into account the risk-benefit ratio.
In regard to treatment, tecovirimat is the only antiviral drug with an EMA-authorized indication for orthopoxvirus infection.
Brincidofovir is not authorized in the EU but has been authorized by the US Food and Drug Administration. However, availability on the European market is limited somewhat by the number of doses.
According to the ECDC, healthcare authorities should provide information about which groups should have priority access to treatment.
The use of antivirals for postexposure prophylaxis should be investigated further. Cidofovir is active in vitro for smallpox but has a pronounced nephrotoxicity profile that makes it unsuitable for first-line treatment.
The ECDC document also proposes an interim case definition for epidemiologic reporting. Further indications will also be provided for the management of monkeypox cases and close contacts. Those infected should remain in isolation until the scabs have fallen off and should, above all, avoid close contact with at-risk or immunosuppressed people as well as pets.
Most infected people can remain at home with supportive care.
Prevention
Close contacts for cases of monkeypox should monitor the development of their symptoms until 21 days have passed from their most recent exposure to the virus.
Healthcare workers should wear appropriate PPE (gloves, water-resistant gowns, FFP2 masks) during screening for suspected cases or when working with confirmed cases. Laboratory staff should also take precautions to avoid exposure in the workplace.
Close contacts of an infected person should not donate blood, organs, or bone marrow for at least 21 days from the last day of exposure.
Finally, the ECDC recommends increasing proactive communication of the risks to increase awareness and provide updates and indications to individuals who are at a greater risk, as well as to the general public. These messages should highlight that monkeypox is spread through close person-to-person contact, especially within the family unit, and also potentially through sexual intercourse. A balance, however, should be maintained between informing the individuals who are at greater risk and communicating that the virus is not easily spread and that the risk for the general population is low.
Human-to-Animal Transmission
A potential risk for human-to-animal transmission exists in Europe; therefore, a close collaboration is required between human and veterinary healthcare authorities, working together to manage domestic animals exposed to the virus and to prevent transmission of the disease to wildlife. To date, the European Food Safety Authority is not aware of any reports of animal infections (domestic or wild) within the EU.
There are still many unknown factors about this outbreak. The ECDC continues to closely monitor any developments and will update the risk assessment as soon as new data and information become available.
If human-to-animal transmission occurs and the virus spreads among animal populations, there is a risk that the disease could become an endemic in Europe. Therefore, human and veterinary healthcare authorities should work together closely to manage cases of domestic animals exposed to the virus and prevent transmission of the disease to wildlife.
This article was translated from Univadis Italy.
Follow Medscape on Facebook, Twitter, Instagram, and YouTube
Source: Read Full Article