Analysis of DNA reveals weapons used by our immune cells to fight tuberculosis
A study led by the Agency for Science, Technology and Research’s (A*STAR) Genome Institute of Singapore (GIS) and Infectious Diseases Labs (ID Labs) has identified a gene, KCNJ15, that is associated with helping our immune system fight tuberculosis (TB), and potentially other infectious diseases. The research was published in Nature Microbiology on 31 January 2022.
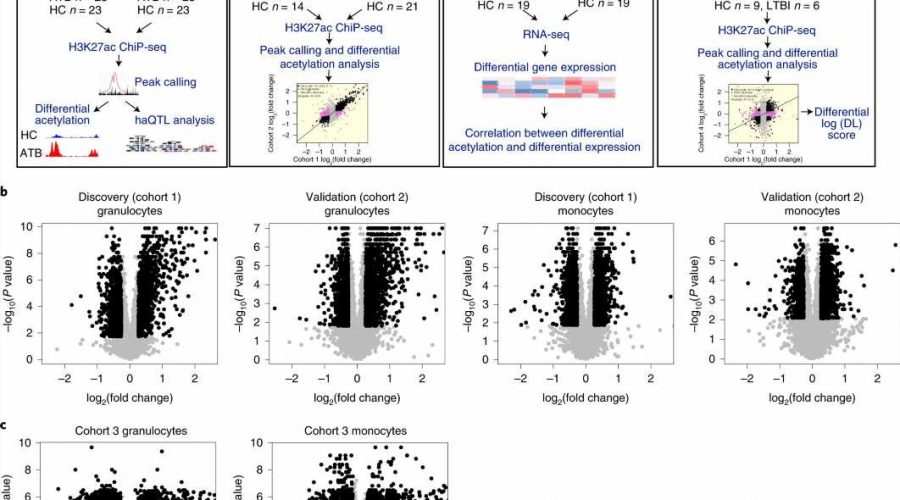
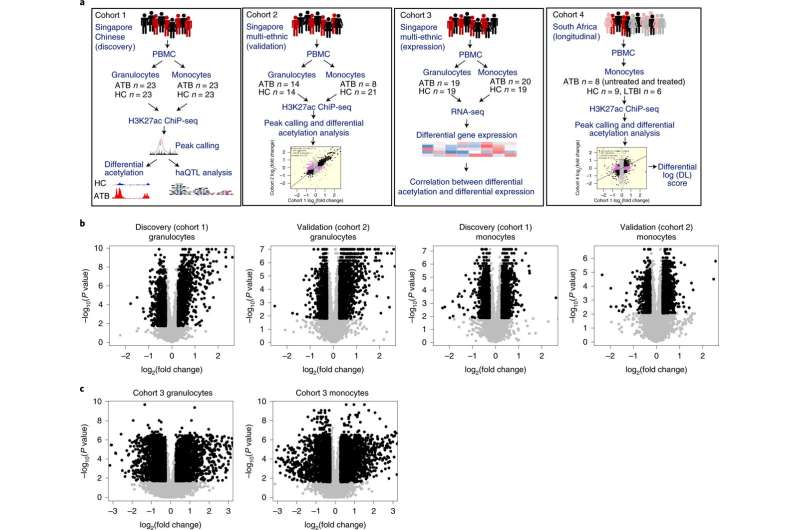
The team used high-throughput genomic technologies to learn how the DNA packaging in blood cells changes when a person has active TB, a bacterial disease that killed one and a half million people globally in 2020. They discovered that TB patients had altered acetylation (a type of chemical modification within the cells) levels at thousands of DNA regions. Their goal was to identify which of these alterations will help to fight TB, and which will help the bacteria to grow.
They discovered that a gene, KCNJ15, which regulates intracellular potassium, was one of the weapons employed by the immune system to fight TB. It increases the level of potassium in the cell, which then causes the cell to self-destruct through a process called apoptosis. This reduces the ability of the bacteria to reproduce inside the cell. Further research could potentially lead to drug development targeting these potassium modulators, to add to the antibiotics currently used against infectious diseases, and may help in building the arsenal against antibiotic-resistant bacteria.
Dr. Shyam Prabhakar, associate director of the spatial and single-cell systems, and principal investigator of the Laboratory of Systems Biology & Data Analytics at GIS, as well as corresponding author of the study, said, “Histone acetylation has been implicated by multiple studies in our immune response to TB and other infectious diseases. However, those studies did not examine the genome in detail to determine which specific parts were affected and to what extent. This is the first study to examine infection-associated histone acetylation changes genome-wide.”
Dr. Amit Singhal, principal investigator at ID Labs, and co-corresponding author of the study, said, “Our study highlights how respiratory infections can affect the chromatin structure and transcriptional program of host cells. These epigenetic alterations might play an essential role in the onset of various respiratory diseases including COVID-19 and can be exploited to develop therapeutic and prophylactic interventions.”
Prof Patrick Tan, executive director of GIS, said, “Proteins such as KCNJ15 could potentially play a role in protecting us from multiple infectious diseases. The success of this study should encourage researchers to perform similar analyses in other infectious diseases, and expand to inflammatory and autoimmune conditions such as type 1 diabetes and rheumatoid arthritis, among others. In addition, results indicate that the role of potassium channels in modulating infection may have been under-appreciated. This is a promising area for future molecular studies and potential drug development.”
Source: Read Full Article