A spike-derived epitope YLQ induces robust polyfunctional cytotoxic T cell responses in COVID-19 recovered individuals
Scientists from Australia have recently deciphered the structural and molecular basis of T cell-mediated recognition of a dominant epitope on the spike protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The findings reveal that the dominant spike-derived epitope induces polyfunctional CD8+ T cell response in coronavirus disease 2019 (COVID-19) recovered individuals. The study is currently available on the bioRxiv* preprint server.
During a viral infection, cytotoxic CD8+ T cells play a vital role in recognizing and eliminating the invading virus. Studies have shown that CD8+ T cells are able to recognize and eliminate SARS-CoV-2 variants in COVID-19 patients efficiently.
Mechanistically, CD8+ T cells recognize viral epitopes presented by human leukocyte antigen (HLA) class I molecules through specific T cell receptors (TCR), leading to the initiation of a cascade of immune reactions that ultimately result in viral clearance. The most prevalent HLA class I molecule in the global population is HLA-A*02:01. A growing pool of evidence has indicated that COVID-19 recovered individuals with HLA-A*02:01 demonstrate a robust CD8+ T cell response to an immunodominant SARS-CoV-2 spike-derived epitope namely YLQ.
The study
The scientists have described the x-ray crystallographic structure of a public TCR – YLQ epitope complex. Moreover, they have characterized the CD8+ T cell response to HLA-A*02:01-presented YLQ epitope in COVID-19 recovered individuals. A public TCR is an epitope-specific TCR shared among unrelated individuals.
CD8+ T cell response to YLQ epitope
The scientists determined the immunogenicity of YLQ epitope in three COVID-19 recovered individuals. By expanding CD8+ T cells against the YLQ epitope, they observed a variable YLQ-specific T cell response and cytokine production between participants. Specifically, only two out of three participants exhibited CD8+ T cells that were able to produce all four cytokines (IFN, TNF, CD107a, and MIP1β); however, only two cytokine-producing CD8+ T cells were detected in the other participant.
Taken together, these observations indicate that after recovery from COVID-19, individuals are capable inducing polyfunctional CD8+ T cell response to YLQ epitope; however, the level of polyfunctionality may differ between individuals.
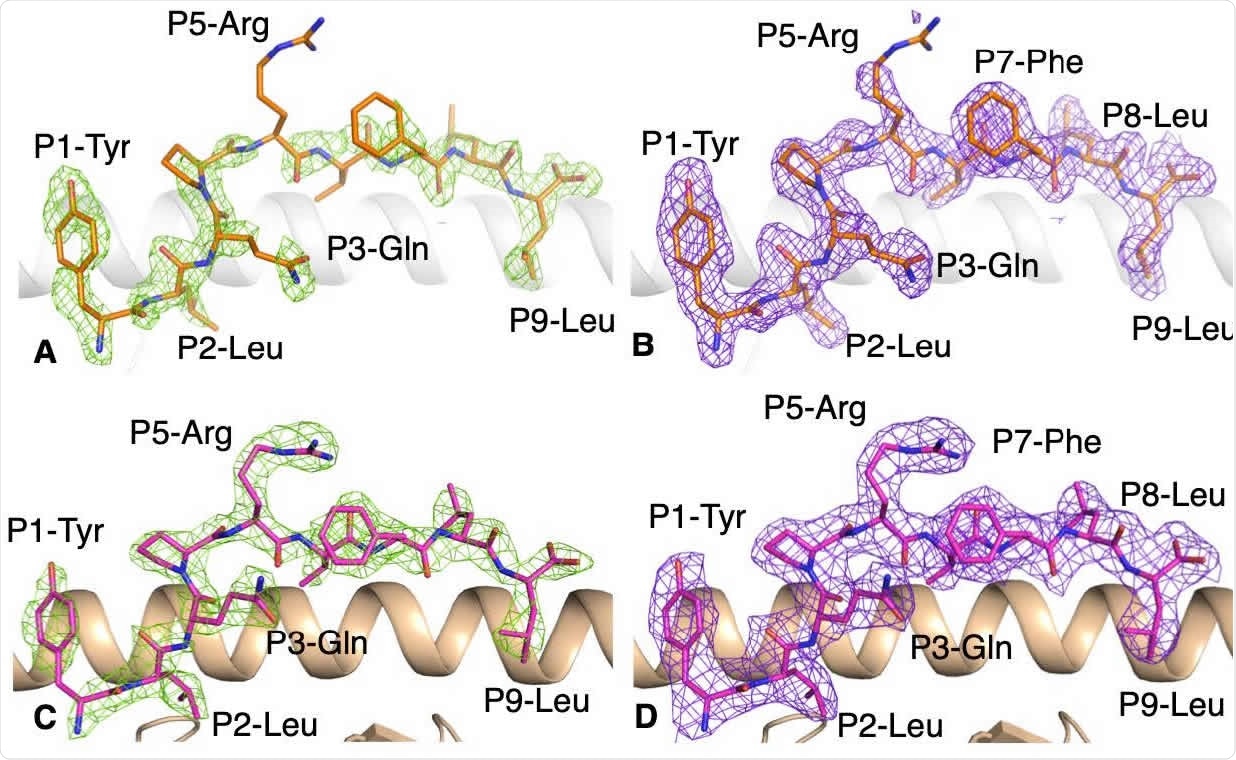
Presentation of YLQ epitope by HLA-A*02:01
The scientists aligned spike sequences of SARS-CoV-2 and seasonal coronaviruses to determine whether the YLQ epitope is conserved between human coronaviruses. They found that the epitope shares only four residues with seasonal coronaviruses. However, they observed that the epitope is highly conserved between different SARS-CoV-2 variants with less than 0.5% of mutations of its residues.
By analyzing the x-ray crystallographic structure of the HLA-A*02:01 – YLQ complex, they revealed that the epitope maintains a stable and rigid conformation in the HLA cleft and that the amino acid residues of the epitope have long side chains that potentially interact with TCRs.
Recognition of YLQ epitope by T cell receptors
From previously available studies, the scientists analyzed TCR sequences of YLQ-specific clonotypes observed in COVID-19 recovered individuals. Their analysis revealed a highly biased TCR repertoire among HLA-A*02:01-positive unrelated individuals. Moreover, they noticed a representation public TCR among unrelated individuals and selected it to further characterize the T cell response to YLQ epitope.
By analyzing the structure of YLQ – public TCR complex, they noticed that the receptor has high binding affinity for the HLA-A*02:01 – YLQ complex. During interaction, the public TCR docks diagonally above the center of YLQ epitope. The alpha-chain of the TCR contributes to about 67% of the interaction. Importantly, minimal structural rearrangements occur during the docking of public TCR onto the HLA-A*02:01 – YLQ complex.
Study significance
The study describes the structural and molecular basis of the interaction between a public TCR and an immunodominant SARS-CoV-2 spike-derived epitope YLQ. In COVID-19 recovered individuals, YLQ-specific T cells exhibit a bias in their TCR repertoire as the alpha-chain of TCR contributes to 67% of the interaction, with the CDR1/2a loops (complementarity determining regions responsible for TCR specificity) contributing to 40% of the total interactions.
As mentioned by the scientists, the YLQ epitope can be used as a potential biomarker of SARS-CoV-2 infection as the epitope is highly immunogenic in most of the COVID-19 recovered individuals and not recognized in healthy individuals. Moreover, the ability to stimulate a robust polyfunctional CD8+ T cell response makes the YLQ epitope a promising target for T cell-based therapies or vaccines against COVID-19.
- Szeto C et al. (2021). Molecular basis of a dominant SARS-CoV-2 Spike-derived epitope presented by HLA-A*02:01 recognised by a public TCR. BioRxiv. https://www.biorxiv.org/content/10.1101/2021.08.15.456333v1
Posted in: Medical Research News | Disease/Infection News
Tags: Amino Acid, Antigen, binding affinity, Biomarker, Cell, Coronavirus, Coronavirus Disease COVID-19, Cytokine, Cytokines, Electron, Human Leukocyte Antigen, Leukocyte, Molecule, Protein, Receptor, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Virus, X-Ray

Written by
Dr. Sanchari Sinha Dutta
Dr. Sanchari Sinha Dutta is a science communicator who believes in spreading the power of science in every corner of the world. She has a Bachelor of Science (B.Sc.) degree and a Master's of Science (M.Sc.) in biology and human physiology. Following her Master's degree, Sanchari went on to study a Ph.D. in human physiology. She has authored more than 10 original research articles, all of which have been published in world renowned international journals.
Source: Read Full Article
