Using donor CAR T cells shows promise in treating myeloma patients in phase I trial
A team of medical specialists working at Memorial Sloan Kettering Cancer Center in New York has found that donated white blood cells can be used effectively as part of CAR T cell therapy to treat myeloma patients.
In their study, published in the journal Nature Medicine, the group gave patients varying amounts of donated CAR T cells as a treatment option in a phase I clinical trial for myeloma, which occurs in plasma cells in bone marrow. Jennifer Brudno and James Kochenderfer with the U.S. National Institutes of Health published a News & Views article in the same journal issue outlining how chimeric antigen receptor (CAR) T cell therapies work and discussing the work done by the team in New York.
CAR T cell therapy involves removing T cells from a cancer patient, modifying them genetically to more specifically target cancer cells, and then injecting them back into the same patient. CAR T cell therapy is highly effective for some patients. The downside is that it is time-consuming to grow the cells after alteration, ruling it out for some patients, and it can be expensive, as well.
In this new effort, the researchers took a different approach, using previously altered cells donated by healthy volunteers. As part of the research, the team in New York ran a phase I clinical trial called UNIVERSAL to find out if the approach is feasible.
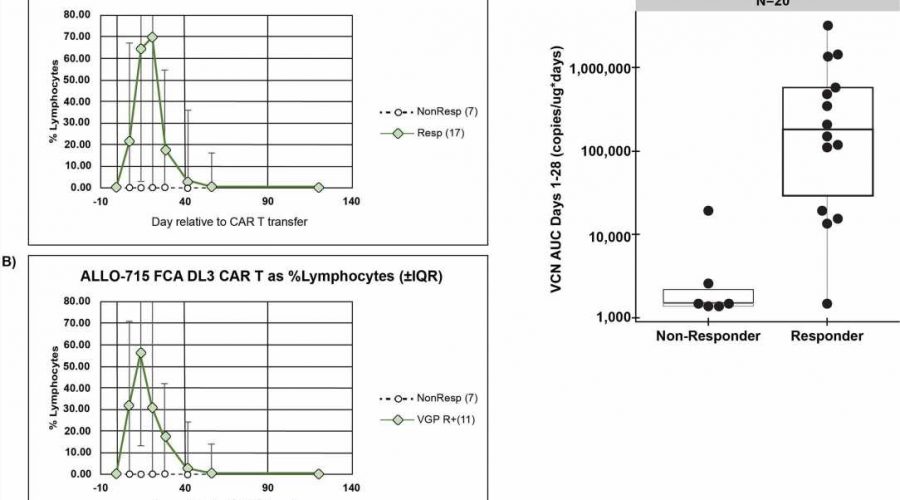
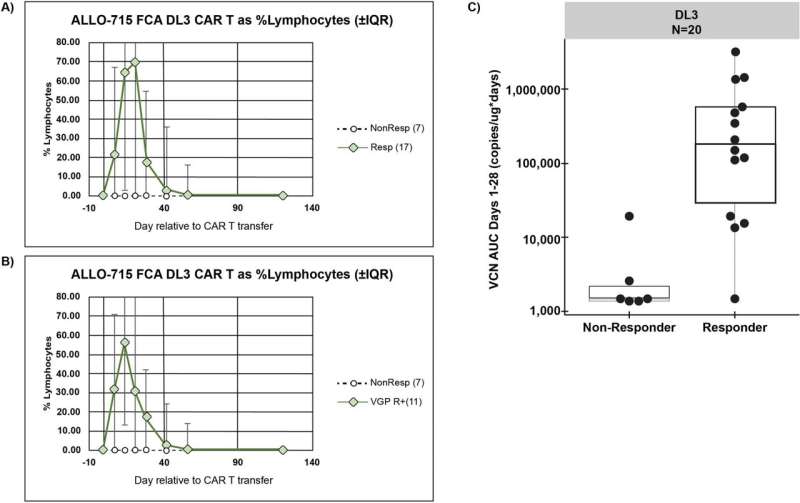
The trial involved giving varying amounts of altered cultivated cells to 43 myeloma patients, each of whom had either not responded well to other treatments or who had relapsed. Also, an additional genetic modification was made to the donated cells to make them less likely to be targeted by the immune system.
Each patient was also given antibodies to reduce the chances of the immune system targeting the donated cells. Testing showed that just over half of the volunteer patients (55%) had a positive response to the therapy. The therapy was also found to be both safe and feasible.
The researchers suggest that their trial was a success and plan to continue their work with the goal of improving results.
More information:
Sham Mailankody et al, Allogeneic BCMA-targeting CAR T cells in relapsed/refractory multiple myeloma: phase 1 UNIVERSAL trial interim results, Nature Medicine (2023). DOI: 10.1038/s41591-022-02182-7
Jennifer N. Brudno et al, Off-the-shelf CAR T cells for multiple myeloma, Nature Medicine (2023). DOI: 10.1038/s41591-022-02195-2
Journal information:
Nature Medicine
Source: Read Full Article