Team elucidates molecular pathway driving maladaptive processes in heart failure, making way for new therapies
Under stress, the human heart struggles to pump blood efficiently through the body. Over time, as the heart overexerts itself trying to make up for this lost efficiency, harmful changes take place. The most worrisome of these changes is a form of maladaptive growth known as cardiac hypertrophy, which eventually leads to heart failure—a condition in which the heart progressively loses its ability to pump blood.
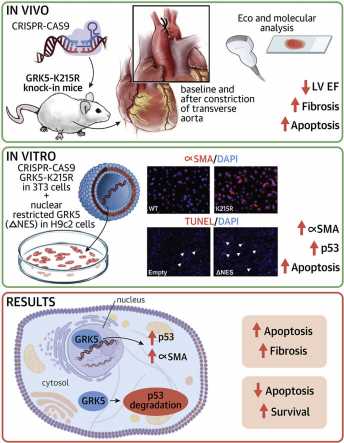
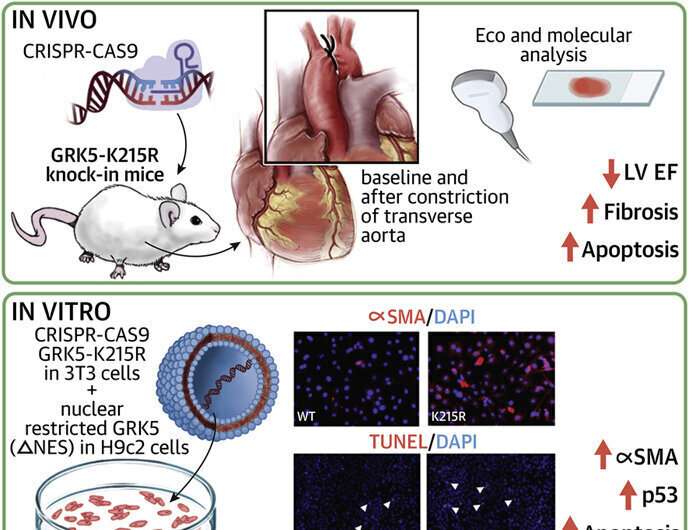
The survival rate for patients with heart failure is poor, creating a need for new therapeutic strategies. Now, scientists at the Lewis Katz School of Medicine at Temple University are ever closer to homing in on a novel approach to treatment, thanks to their groundbreaking research on the G protein-coupled receptor kinase 5 (GRK5) signaling molecule. The Temple researchers show in new work that GRK5, already known to influence cardiac hypertrophy, promotes maladaptive cardiac remodeling and progressive decline in heart function by keeping heart cells alive. In cell and animal models, GRK5 was found to both specifically induce p53—a protein that normally induces apoptosis, a form of cell death—and regulate the activity of fibroblasts, which contribute to abnormal heart growth and functional loss.
The study, published online in the Journal of the American College of Cardiology: Basic to Translational Science, is the first to link cardiac hypertrophy and GRK5 to p53, which is better known for its involvement in cancer. The new work also demonstrates the ability of GRK5 to operate in the heart cell nucleus via a so-called non-canonical, or alternate, pathway.
“In this new study, we have dissected the mechanisms of action of GRK5, revealing the importance of its catalytic activity in influencing myocardial function through direct regulation of cardiomyocytes and fibroblasts,” said Walter J. Koch, Ph.D., W.W. Smith Endowed Chair in Cardiovascular Medicine, Professor and Chair of the Department of Cardiovascular Sciences, and Director of the Center for Translational Medicine at the Katz School of Medicine, as well as corresponding author on the new study.
“GRK5 is a multifunctional protein expressed in several cell types, including cardiomyocytes and cancer cells,” explained Alessandro Cannavo, Ph.D., Associate Professor in the Department of Translational Medical Sciences at Federico II University of Naples and senior author on the study. “Furthermore, GRK5 can be either a protective protein or a toxic protein, depending on whether it is within or outside of the cell nucleus.”
Dr. Cannavo, who started this study while a fellow in Dr. Koch’s lab at the Katz School of Medicine before his faculty position at Naples, and colleagues carried out their investigation in cells that expressed a form of GRK5 rendered incapable of entering the nucleus and in mice that expressed a mutated, catalytically inactive version of the molecule known as GRK5-K215R. Experiments in vitro showed that when GRK5 was prevented from entering the nucleus, p53 levels were increased and cell death was enhanced. In the mouse model, expression of catalytically inactive GRK5 was associated with marked decreases in cardiac function. Moreover, in fibroblasts, the K215R mutation promoted the transition into a myofibroblast phenotype, which is linked to maladaptive remodeling and decreased function of the heart.
“Our data indicate that the influence of GRK5 on cardiac hypertrophy is due not just to its catalytic activity but also to its activity in the heart cell nucleus,” Dr. Koch said. “This suggest that therapeutically, rather than inhibiting GRK5 globally, within and outside of the nucleus, maladaptation processes would be more effectively blocked through the use of drugs that specifically prevent GRK5 from getting into the nucleus.”
Source: Read Full Article