Representation of Skin of Color Patients in Laser Trials Low
Patients with darker skin phototypes are “poorly represented” in randomized controlled trials of laser and light treatments for cosmetic dermatologic conditions, according to a systematic review of the literature, the authors reported.
“While there broadly appears to be skin of color representation [in such studies], a more granular understanding of the data shows a large discrepancy in representation between ‘lighter’ and ‘darker’ skin of color patients,” Priya Manjaly and associates wrote in the Journal of Cosmetic Dermatology.
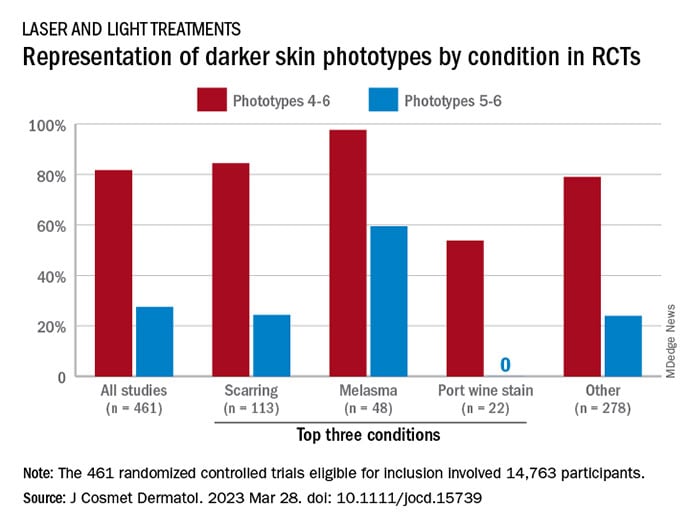
Among the 461 randomized controlled trials (RCTs) eligible for inclusion, most (81.7%) included participants with skin phototypes 4-6, which is considered skin of color. When only phototypes 5 and 6 were included, however, representation in studies involving laser and light devices was only 27.5%, said Ms. Manjaly, a research fellow in the department of dermatology at Boston University, and associates.
“This trend of excluding darker skin phototypes persisted when the results were stratified by condition, laser of study, study location, journal type, and funding source,” the investigators noted.
RCTs of laser/light devices for scarring, the most common dermatologic condition represented, included phototypes 5 and 6 in 24.4% of studies, compared with 84.4% for phototypes 4-6. The gap was smaller for melasma, but not for port wine stains. Among the devices examined, RCTs of diode lasers and intense pulsed light had the smallest gaps between inclusion of the two groups of phototypes, while pulsed-dye laser studies had the largest, they reported.
Stratification by journal showed the largest gap in studies published by Lasers in Medical Science and the smallest gap coming from Lasers in Surgery and Medicine. Funding was not specified for the majority of the eligible device RCTs, but those funded by industry had the smallest discrepancy between types 5-6 and types 4-6 and those supported by foundations/nonprofits the largest, Ms. Manjaly and associates said.
“With projections estimating that more than 50% of the U.S. population is set to identify as Hispanic or nonwhite by 2045 … the lack of information has important consequences for clinical practice, as clinicians are unable to counsel patients on the efficacy and possible complications of various devices in patient with skin of color,” they wrote.
The investigators did not declare any conflicts of interest or funding sources.
This article originally appeared on MDedge.com, part of the Medscape Professional Network.
Source: Read Full Article
