A molecular traffic light for infectious disease testing
Home test kits to check for COVID-19 spike proteins and anti-COVID-19 antibodies are fast and simple to use but lack the sensitivity and accuracy of laboratory tests. Researchers from Eindhoven University of Technology with Utrecht University have developed a new type of sensor that combines the sensitivity and accuracy of current laboratory-based measurements with the speed and low-cost of current home tests. The new sensor uses a “glow-in-the-dark” signal to indicate the presence of tiny amounts proteins and anti-drug antibodies, as well as COVID-19 spike proteins and antibodies in blood. This new research has been published in Nature Communications.
“There is an increasing demand for medical tests in light of the current COVID-19 pandemic that yield fast and accurate results, are cheap, and can ideally be done by anyone, anywhere,” notes Maarten Merkx from the institute for Complex Molecular Systems (ICMS) at TU/e and research lead for the new study.
“Of course, self-tests are widely available, but the fact remains that these are still less sensitive and accurate than laboratory tests. But you may be waiting one or more days for the result from a laboratory test, and that can be too long,” adds Merkx.
Another issue with these so-called point-of-use home tests is that they are not accurate enough. “Output from a corona self-test is a simple yes or no, positive or negative,” says Merkx, who is also based at the Department of Biomedical Engineering at TU/e. “For some applications, it would be better if we could quantify the concentration of proteins or antibodies in a sample.”
With all of this in mind, Merkx and the research team (with Yan Ni, Bas Rosier, and Eva van Aalen as the lead authors of the paper) developed a new test approach with the accuracy and sensitivity of current laboratory-based methods and the speed and low-cost of existing point-of-use tests.
The key to detecting and indicating the presence of biomarkers or molecules of interest is bioluminescence, the process that organisms such as fireflies use to produce light. “Our new sensor is based on bioluminescence, which we have used extensively in our previous research. If a certain protein or antibody is present in the sample, special proteins called luciferase enzymes will emit light,” notes Merkx. “In other words, we look for a ‘glow-in-the-dark’ response.”

Molecular traffic light
A critical part of the new sensor is that it is always emitting light via bioluminescence. So how do the researchers know if a biomarker has been detected then? Well, they look for a color change in the light emitted, just like a molecular traffic light.
“When the device contains no biomarkers of interest, one type of luciferase enzyme emits green light. It’s always emitting green light, and it is not affected when a sample containing biomarkers is added,” notes Merkx. “But it’s a second luciferase enzyme that holds the key to detection.”
The researchers split the second type of luciferase enzyme into two parts. Each part by itself does nothing, and the enzyme only becomes active when the two parts are joined together. Each part of the luciferase is chemically connected to a pair of antibodies that recognize different parts of a biomarker. When a biomarker is present in a test sample, the antibodies bind to the biomarker, and in the process this brings the two parts of the luciferase together, leading to the emission of blue light.
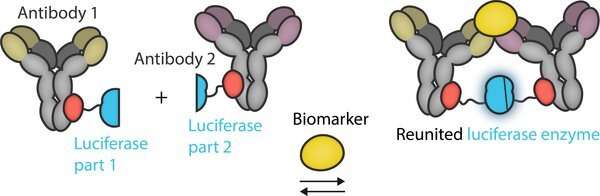
Ratiometric detection
“To quantify the concentration of biomarker, we measure the ratio of the blue to green light. The more blue light that is visible, the greater the concentration of biomarkers in the sample. It’s even possible to record this ratio using a camera on a smartphone, provided the sample is placed in a black plastic container that blocks out any ambient light,” says Merkx.
This is known as ratiometric detection, which is reflected in the name of the test approach RAPPID—ratiometric plug-and-plug immunodiagnostics.
The researchers tested the new sensing approach on a variety of biomarkers, including one to detect bacterial and viral infections (C-reactive protein), using 40 patient samples in collaboration with the Rijnstate hospital in Arnhem.
Broad applications
The RAPPID testing platform is definitely rapid in nature, and with a suitable sample holder can be used in conjunction with a smartphone, meaning that it has the potential to be used by anyone, anywhere—exactly what Merkx and the team are aiming for. However, some work still needs to be done before his new testing platform will become available to the wider public.
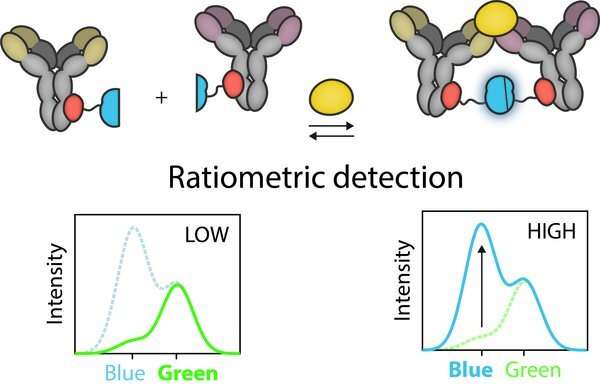
“We envisage that this testing platform could be used for a broad range of applications such as in rapid screening and testing, for therapeutic antibody-drug monitoring associated with conditions like rheumatoid arthritis and inflammatory bowel disease, and for the rapid detection of infectious diseases that could be associated with future epidemics or pandemics,” adds Merkx.
Source: Read Full Article
